Scientists from the National Institutes of Health, USA, have recently demonstrated the immunogenicity and protective efficacy of two vesicular stomatitis virus (VSV)-based coronavirus disease 2019 (COVID-19) vaccines in hamsters. Their study, which is currently available on the medRxiv* preprint server, has described that the intranasal administration of single-dose vaccine provides rapid protection against symptomatic COVID-19 and that the vaccine is effective against different variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The recombinant VSV-based platform has been used previously to develop vaccines against Ebola, Nipah, and Lassa viruses. This platform has several advantages over other commonly used viral vector-based platforms, including the adenoviral vector-based vaccine platform. For example, a single-dose immunization with VSV-based vaccines has been shown to induce strong immune responses in hosts within a short period of time (7 – 10 days post-immunization). Moreover, studies have shown that VSV vaccines induce equivalent immune responses when administered intramuscularly and intranasally. Another potential advantage is the lack of preexisting immunity against VSV in the human population.
In the current study, scientists have described the immunogenicity and protective efficacy of two VSV-based COVID-19 vaccines in hamsters. They have tested both intramuscular and intranasal routes of administration.
In the monovalent vaccine, they have used a SARS-CoV-2 spike variant with a cytoplasmic tail deletion. In the bivalent vaccine, they have used full-length SARS-CoV-2 spike and the Ebola virus glycoprotein.
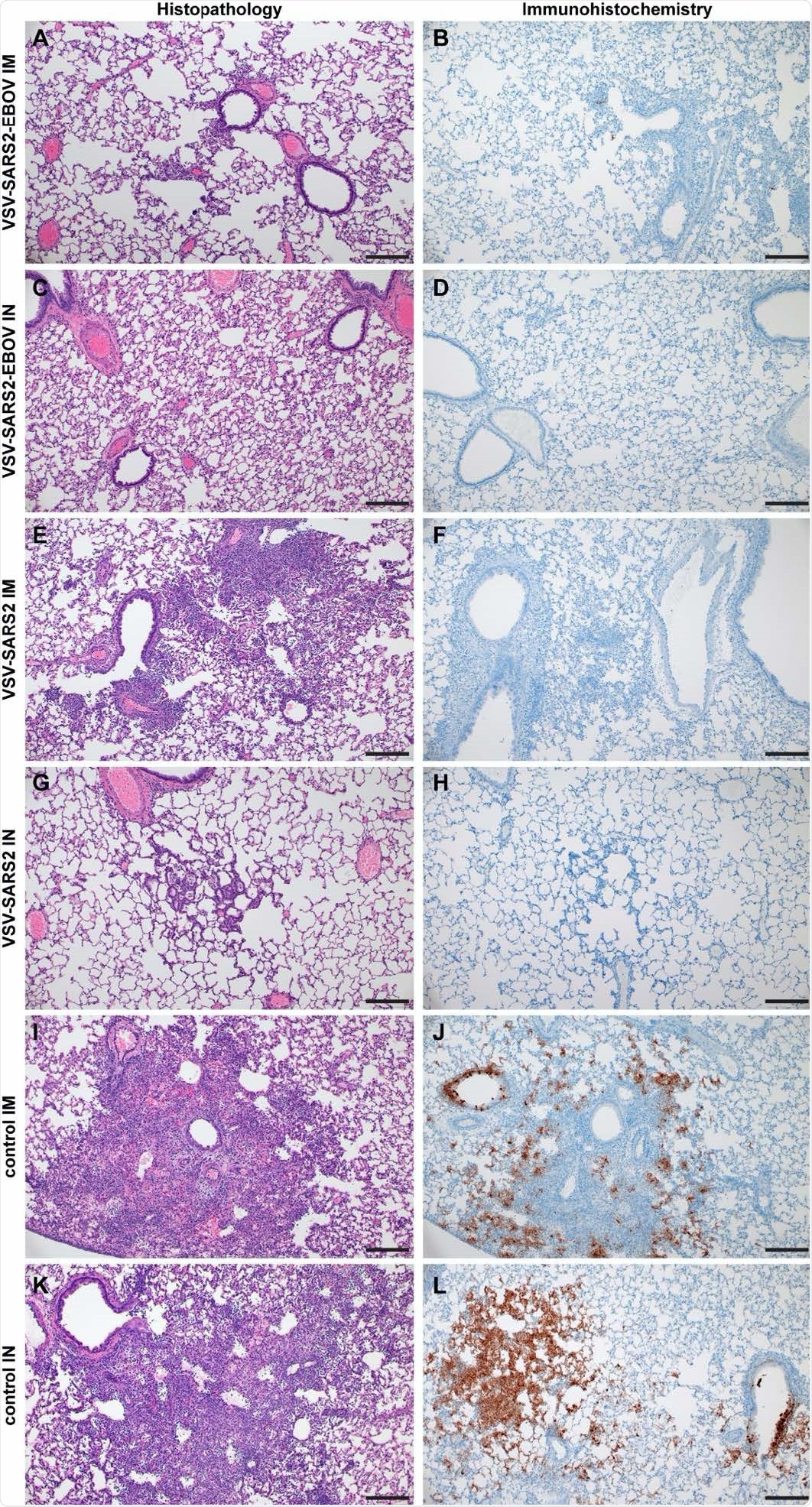
Histopathology and Immunohistochemistry of hamster lungs with challenge 10 DPV. Hamsters were vaccinated 10 days prior to challenge with SARS-CoV-2 WA1. At 4 days after challenge lung samples were collected and stained with H&E or anti-SARS-CoV-2 nucleocapsid (N) antibody for IHC. (A) Rare foci of minimal to mild interstitial pneumonia with mild alveolar spillover. (B) Rare type I pneumocyte immunoreactivity. (C) Lack of notable pulmonary histopathology. (D) No immunoreactivity to SARS-CoV-2 N. (E) Focus of mild to moderate broncho-interstitial pneumonia with perivascular leukocyte cuffing. (F) Limited type I pneumocyte immunoreactivity. (G) Rare foci of minimal to mild interstitial pneumonia with type II pneumocyte hyperplasia. (H) No immunoreactivity to SARS-CoV-2 N. (I) Focus of moderate to severe bronchointerstitial pneumonia with disruption of pulmonary architecture by degenerate and non-degenerate neutrophils, macrophages and cellular debris accompanied with perivascular and pulmonary edema. (J) Abundant immunoreactivity to SARS-CoV-2 N in the columnar epithelium of bronchioles, type I pneumocytes and alveolar macrophages. (K) Moderate broncho-interstitial pneumonia with influx of moderate to numerous leukocytes and limited pulmonary edema. (L) Abundant immunoreactivity to SARS-CoV-2 N in bronchiolar epithelium, type I and II pneumocytes and within cellular debris. (200x, bar = 50 μM).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study design
The scientists administered Syrian golden hamsters with a single dose of monovalent or bivalent vaccine through the intramuscular or intranasal route. They collected samples from vaccinated hamsters at days 3, 10, 21, and 38 post-immunization for immunogenicity analysis. For efficacy testing, vaccinated hamsters were challenged with SARS-CoV-2 at days 10 and 28 post-vaccination.
Vaccine-induced antibody response
The analysis conducted on day 10 post-vaccination revealed that Both vaccines are capable of inducing detectable levels of anti-SARS-CoV-2 antibodies irrespective of the route of administration. Compared to the monovalent vaccine, the bivalent vaccine showed a higher ability to induce robust and long-lasting antibody responses in hamsters when administered intranasally.
Vaccine-induced cellular immune response
The analysis conducted on day 3 post-vaccination revealed a high percentage of activated CD4+ T cells in hamsters immunized intramuscularly. The highest level of CD4+ T cell response was observed at day 10 in hamsters immunized intranasally with the bivalent vaccine. Overall, intramuscular vaccination caused a rapid CD4+ T cell and natural killer cell response. In contrast, intranasal vaccination resulted in a rapid CD8+ T cell response in the lungs.
The analysis of spleen and peripheral blood mononuclear cells revealed that both intramuscular and intranasal vaccination induces peak levels of CD4+ T cell responses in the spleen at day 10, with intranasal vaccination inducing more CD4+ T cells at day 38 and intramuscular vaccination inducing more CD8+ T cells at days 3 and 10 post-immunization. A similar pattern of cellular immunity was observed in peripheral blood mononuclear cells of vaccinated hamsters.
Protection against wildtype SARS-CoV-2
Compared to unvaccinated control hamsters, vaccinated hamsters showed significantly lower lung viral loads and absence of pulmonary lesions post-viral challenge. A significantly higher level of spike-specific antibodies was detected in intranasally immunized hamsters that were challenged with the wild-type virus at day 28 post-immunization. Compared to monovalent vaccine, the bivalent vaccine showed significantly higher neutralizing ability when administered intranasally.
The analysis conducted on hamsters challenged at day 10 post-vaccination revealed that both vaccines provide significant protection against lung viral load and interstitial pneumonia when administered intranasally. Importantly, an equivalent level of protection was observed in hamsters immunized intramuscularly with the bivalent vaccine.
When administered intranasally, both vaccines induced robust anti-spike immune responses with potent neutralizing activity. Similar antibody response was observed in hamsters immunized intramuscularly with the bivalent vaccine.
Protection against SARS-CoV-2 variants
Both vaccines showed significant protection against viral load and lung lesions in hamsters that were challenged with B.1.1.7 variant at day 10 post-intranasal vaccination. Similarly, only intranasally immunized hamsters showed significantly lower lung viral loads and interstitial pneumonia following infection with B.1.351 variant. The highest protection was observed in hamsters intranasally immunized with the bivalent vaccine.
Regarding anti-spike antibody responses, a significantly higher binding antibody titer was observed in hamsters that were immunized intranasally with the bivalent vaccine and challenged with the B.1.351 variant. In contrast, the highest neutralizing antibody titer against the B.1.351 variant was observed in hamsters that were intranasally immunized with the monovalent vaccine.
Study significance
The single-dose VSV-based COVID-19 vaccines developed by the scientists demonstrate high antiviral efficacy against wild-type SARS-CoV-2 and its variants B.1.1.7 and B.1.351 within 10 days of administration. Both vaccines provide the highest protection when administered intranasally.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources