The coronavirus disease 2019 (COVID-19) continues to afflict human communities across the globe. The best strategy to prevent severe symptoms of COVID-19 appears to be through establishing herd immunity with effective and sustainable vaccines.
Unfortunately, despite remarkable efficacy in trials, the knowledge of durability of immune memory post-vaccination with inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains obscure. Moreover, the rapid emergence of novel SARS-CoV-2 variants of concern (VOCs) has diminished the efficacy of SARS-CoV-2 vaccines.
The presence of neutralizing antibodies (NAbs) against SARS-CoV-2—indicative of the protective immunity after two doses of vaccination or an infection—has shown a rapid decline with time.
Some patients with B cell deficiency (inherited or treatment-induced) were able to recover from COVID-19, which was suggestive of a potential role of cellular responses against SARS-CoV-2 infection.
Furthermore, inactivated vaccines triggered the generation of SARS-CoV-2 specific memory B cells, which is essential for a rapid and robust recall of protective responses against viral infection.
T cells also play a crucial role in coordinating the adaptive immune responses and as effectors against viral infection. But there is very little information available as to how long can these immune responses sustain.
To investigate the immune memory sustainability of SARS-CoV-2-specific B cells and T cells, stimulated by the inactivated vaccine, and the potential need for a third booster dose in healthcare workers (HCWs), a non-randomized trial was undertaken by researchers at Sun Yat-sen University in Guangzhou, China. the research is posted online on the medRxiv* preprint server.
This non-randomized trial included 63 HCWs who were selected from a prospective cohort at FAH-SYSU. Among these, 50 HCWs workers volunteered to receive a third booster shot of the inactivated vaccine after six months (d180) of the prime vaccination.
These HCWs had previously received the inactivated SARS-CoV-2 vaccine BBIBP-CorV (BBIBP-CorV, Sinopharm, Beijing) in the morning (9 am-11 am) or afternoon (15 pm-17 pm) on days (d) 0 and 28, respectively. An equal number of members (25 each) were assigned to morning or afternoon groups, and the vaccinating slots were maintained through all three doses. Blood samples were collected into the heparinized tubes and processed immediately after sample collection, at different time-points d0 (T1), d14 (T2), d21 (T3), d28 (T4), d56 (T5), d180 (T6), d187 (T7), d194 (T8) and d208 (T9). The positive control comprised convalescent patients who had recovered from SARS-CoV-2 infection.
Neutralizing antibodies (NAbs) against SARS-CoV-2 in the serum of each volunteer was measured by Chemifluorescence immunoassay longitudinally. SARS-CoV-2-specific memory B cells and CD4+ T cells and their responses were measured by flow cytometry of spike- and receptor-binding domain (RBD)-specific B cells. The cellular response was measured by IFN-γ ELISpot.
Findings
Even though the standard two-dose vaccinations elicited Nabs—that dropped from a peak of 31.2 AU/ml to 9.2 AU/ml, five months after the second vaccination dose—spike-specific memory B and T cells were still detectable, which formed the basis for a quick recall response.
The faded humoral immune response was robustly elevated to 66.8 AU/ml – by 7.2 folds, one week after the third dose, along with abundant spike-specific circulating follicular helper T cells in parallel.
In addition, spike-specific CD4+ and CD8+ T cells were magnanimously elevated – by 5.9 and 2.7 folds, respectively.
Another noteworthy finding in this trial was that the HCWs with low serological response to two doses were not truly “no responders.” In fact, they harbored a complete immune memory that could be promptly recalled by a third dose, even five months after the second vaccination dose.
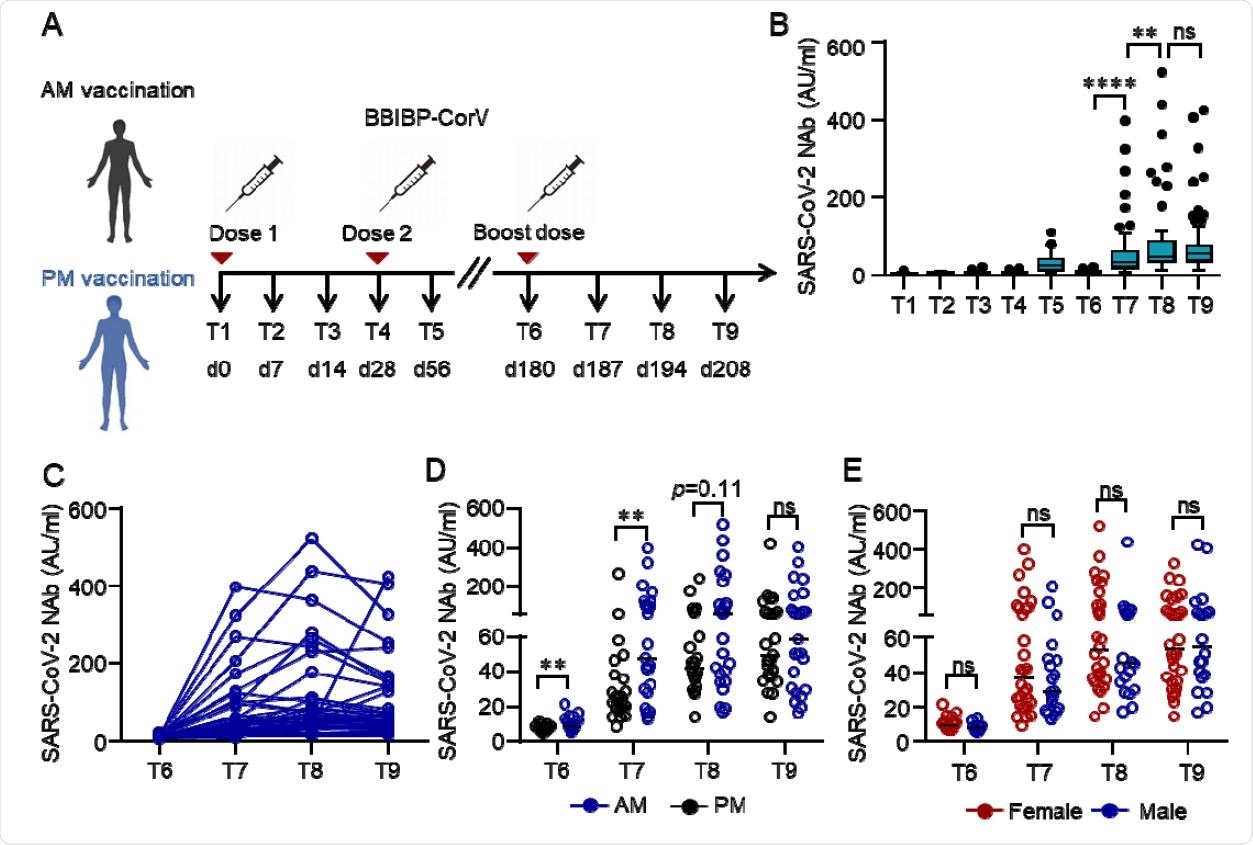
Humoral response to the third dose of inactivated SARS-CoV-2 vaccine BBIBP-CorV. (A) Protocol of the prospective cohort and the non-randomized trial. Healthcare workers (HCWs) were recruited from a perspective cohort who received two doses of an inactivated vaccine either in the morning (n=25) or afternoon (n=25) on day (d) 0 and d28 . They were assigned to morning or afternoon vaccination to receive a third dose of the inactivated vaccine on day 180 according to their previous vaccinating time. Blood samples were collected on different time points d0 (T1), d14 (T2), d21 (T3), d28 (T4), d56 (T5), d180 (T6), d187 (T7), d194 (T8) and d208 (T9). (B-E) Neutralizing antibodies (NAbs) against SARS-CoV-2 in the serum of each volunteer was measured by Chemifluorescence Assay longitudinally. The concentration of NAbs in the sera at different time points was summarized and shown in the box plot (B). The longitudinal changes of NAbs in the sera before and after the third dose of vaccination (C). NAbs concentrations in the sera from the morning or afternoon group at different time points before and after the third dose of vaccine. Data were summarized and shown as dot plot (D). NAbs concentrations in the sera of female or male at different time points before and after the third dose of vaccination were summarized and shown as dot plot (E). Medians of the data were shown. **p<0.01, ****p<0.0001. Comparisons were done by Wilcoxon rank sum test. Ns: not significant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Significance
Data from this study validate the generation of long-term immunological memory by inactivated vaccine. This has bearing for future strategies for frontline HCWs who face high exposure rates and dangers from SARS-CoV-2; individuals with low serological response to the second dose of vaccine; and immuno-compromised patients – who would benefit with a third booster dose. The induction of SARS-CoV-2-specific memory B cells and T cells could represent key factors in the development of long-term protective immunity against SARS-CoV-2 infection, even with predictions of decayed humoral immunity as time elapses after vaccination and the emergence of variants of concern (VOCs).
Limitations of the study
These findings need to be confirmed through other independent studies to demonstrate the clinical relevance of the above-mentioned immunological responses by inactivated vaccination.
Conclusions
Five months after the second vaccination dose, while the Nabs declined substantially, SARS-CoV-2-specific memory B, CD4+, and CD8+ T cells persisted in the peripheral blood. These results extended even to the participants who were seronegative after two doses of the inactivated vaccine. Besides, the third vaccination dose caused an intensive recall of humoral and cellular immune responses in all participants and promised long-lasting preventive benefits.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Liu Y, Zeng Q, Deng C, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. September 2021. doi 10.1101/2021.09.12.21263373, https://www.medrxiv.org/content/10.1101/2021.09.12.21263373v1
- Peer reviewed and published scientific report.
Liu, Yihao, Qin Zeng, Caiguanxi Deng, Mengyuan Li, Liubing Li, Dayue Liu, Ming Liu, et al. 2022. “Robust Induction of B Cell and T Cell Responses by a Third Dose of Inactivated SARS-CoV-2 Vaccine.” Cell Discovery 8 (1): 1–13. https://doi.org/10.1038/s41421-022-00373-7. https://www.nature.com/articles/s41421-022-00373-7.