Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus, causes coronavirus disease 2019 (COVID-19), which is a multisystem disease affecting the respiratory tract. Data available so far shows endothelial injury caused by hyperactivation of the immune system as a major underlying molecular mechanism for COVID-19 severity and mortality. In addition, this endothelial involvement leads to many hemostasis abnormalities in severe COVID-19 patients.
Thus, in addition to elevated levels of pro- and anti-inflammatory mediators such as interleukin (IL)-6, IL-10, IL-2R, and tumor necrosis factor-α (TNF-α), increased levels of D-dimer, fibrinogen, and prolonged prothrombin time (PT) have also been found in severe COVID-19 patients. These are clinically relevant because abnormal levels of D-dimer are associated with 28-day mortality in COVID-19 patients. Also, post-mortem studies show the presence of micro-thrombi and capillarostasis in the lungs of the patients. The high incidence of thrombotic events in severe COVID-19 patients indicates a possible role of the contact system pathway in COVID-19 pathology.
FXII is activated in severe COVID-19 patients
Researchers from Europe recently demonstrated altered levels of factor XII (FXII) and its activation products in 2 independent cohorts of critically ill patients with COVID-19 compared to patients suffering from severe acute respiratory distress syndrome caused by the influenza virus (acute respiratory distress syndrome (ARDS)-influenza). This study is published on the preprint server, bioRxiv*.
FXII is a serine protease in the contact-phase system of blood coagulation that circulates in plasma as a single-chain zymogen.
On coming into contact with anionic surfaces like kaolin, neutrophil extracellular traps, extracellular RNA from damaged cells, or polyphosphates from activated platelets, FXII is autoactivated to αFXIIa.
Overall, activation of the contact-phase system leads to increased thrombin and fibrin production, although FXIIa/PKa-mediated conversion of plasminogen to plasmin may have a mild effect on fibrinolysis.
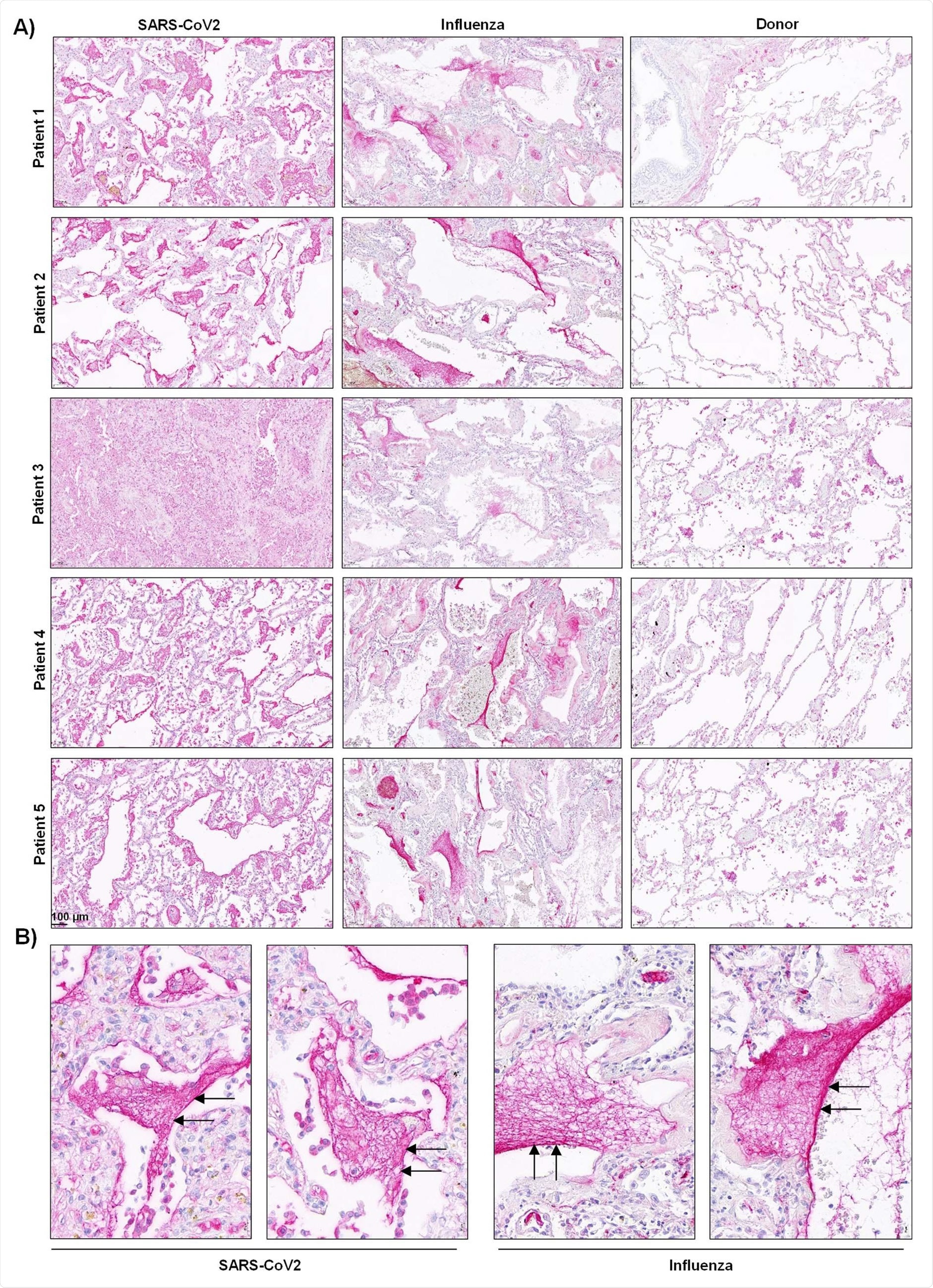
Activation of the contact phase system in plasma of critically ill COVID-19 patients. A,C) Western blot analysis (left panels) of factor XII (FXII) A) and high molecular weight kininogen (HK) C) in plasma from moderate and severe COVID-19 patients (infected with SARS-CoV2) and donors. Four out of 15 moderate and severe COVID-19 patients and 3 out of 15 donors are demonstrated. Rights panels show the specificity of the antibodies used. B, D) Densitometric analysis of A) and C), respectively. COVID-19 moderate/severe n=15, donor n=15. E) PKa-like activity in plasma from moderate (n=14) and severe (n=14) COVID-19 patients and donors (n=15). F) Correlation between the levels of intact HK and PKa-like activity in plasma of severe Covid-19 patients. n=14. Correlation is performed using Spearman’s rank correlation coefficient. *p<0.05, **p<0.01, ***p<0.001.Data in B), D), and E) are shown as mean+/-SD.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Fibrinogen and FXIIa regulate fibrin network density in COVID-19
The researchers also reported rapid consumption of FXII in the plasma of COVID-19 patients, but not in plasma from ARDS-influenza, which is compatible with the above data. Interestingly, the kaolin clotting time was not prolonged in COVID-19 patients compared to that in ARDS-influenza patients.
“Elevated levels of fibrinogen were reported to contribute to the faster fibrin formation and increased fibrin network density, strength, and stability.”
With the help of confocal and electron microscopy, the researchers showed that increased FXII activation rate, along with elevated fibrinogen levels, drives the formation of fibrinolysis-resistant clots with thin fibers and small pores in COVID-19 patients.
“Clots generated from COVID-19 plasma exhibited higher packing density, small pores and were built of thin fibers.”
Accordingly, clot lysis was observed in 30% of COVID-19 patients and 84% of ARDS influenza patients. On analyzing lung tissue sections of COVID-19 patients, extensive extra- and intra-vascular compact fibrin deposits were revealed.
Findings help establish a model for future studies on the role of altered fibrin clot structure in COVID-19-related thrombosis
On the basis of current and previous findings, the uncontrolled response of defense mechanisms, including the immune and coagulation system, constitute the underlying mechanism for severe SARS-CoV-2 infection.
Abnormalities in plasma composition, blood immune cells due to virus-mediated cell damage, and release of intracellular debris all favor the activation of FXII. Apart from high levels of fibrinogen, FXIIa leads to pathologic thrombus formation not only through thrombin generation but also via the formation of compact and lysis-resistant clots.
Overall, the results of the study indicate that increased fibrinogen levels and increased FXII activation rate drive thrombosis and thrombolysis resistance through enhanced thrombus formation and stability in COVID-19 patients. Thus, this study establishes a model for future studies on the role of altered fibrin clot structure in thrombosis and thrombolysis in severe COVID-19 patients.
“Whether the interaction of FXII/FXIIa with fibrinogen can interfere with the binding of t-PA to fibrin and thereby inhibits fibrinolysis warrants further investigation.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Altered fibrin clot structure contributes to thrombosis risk in severe COVID-19 Malgorzata Wygrecka, Anna Birnhuber, Benjamin Seeliger, Laura Michalick, Oleg Pak, Astrid-Solveig Schultz, Fabian Schramm, Martin Zacharias, Gregor Gorkiewicz, Sascha David, Tobias Welte, Julius J. Schmidt, Norbert Weissmann, Ralph T. Schermuly, Guillermo Barreto, Liliana Schaefer, Philipp Markart, Markus C. Brack, Stefan Hippenstiel, Florian Kurth, Leif E. Sander, Martin Witzenrath, Wolfgang M. Kuebler, Grazyna Kwapiszewska, Klaus T. Preissner, bioRxiv, 2021.09.17.460777; doi: https://doi.org/10.1101/2021.09.17.460777, https://www.biorxiv.org/content/10.1101/2021.09.17.460777v1
- Peer reviewed and published scientific report.
Wygrecka, Malgorzata, Anna Birnhuber, Benjamin Seeliger, Laura Michalick, Oleg Pak, Astrid-Solveig Schultz, Fabian Schramm, et al. 2022. “Altered Fibrin Clot Structure and Dysregulated Fibrinolysis Contribute to Thrombosis Risk in Severe COVID-19.” Blood Advances 6 (3): 1074–87. https://doi.org/10.1182/bloodadvances.2021004816. https://ashpublications.org/bloodadvances/article/6/3/1074/482891/Altered-fibrin-clot-structure-and-dysregulated.