Researchers in Japan have conducted a study showing that the vaccine developed by Pfizer-BioNTech to protect against coronavirus disease 2019 (COVID-19) seems to be adequately effective against the B.1.621 (mu) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The mu variant, which has been classified by the World Health Organization (WHO) as a variant of interest, has recently triggered concerns after reports indicated that it exhibits increased immune escape capabilities compared with other variants of concern and interest.
A team from Yokohama City University School of Medicine and Yokohama City University Hospital has conducted a study showing that Pfizer-BioNTech’s BNT162b2 vaccine is 76% effective at neutralizing the mu variant.
The dual monoclonal antibody cocktail containing casirivimab and imdevimab also effectively neutralized the variant.
The study also found that the mu lineage causes cell-to-cell fusion, which can be a factor in promoting escape from vaccine-induced humoral (antibody) immunity.
However, Kei Miyakawa and colleagues say that although mu could become problematic if it replaces the B.1.617.2 (delta) lineage as the predominant variant, the currently available vaccines and casirivimab plus imdevimab cocktail will still be effective at protecting the majority of individuals.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The concerns surrounding mu and other SARS-CoV-2 variants
Since the COVID-19 outbreak first began in late December 2019, the rapid spread and evolution of the causative agent SARS-CoV-2 has led to the emergence of several variants that exhibit increased transmissibility and, in some cases, the ability to escape vaccine- or infection-induced immunity.
With vaccines representing the most promising approach to gaining herd immunity and curtailing the pandemic, no option remains other than to continue immunization programs while preparing for the emergence of immune escape variants.
The WHO has so far classified the B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma) and B.1.617.2 (delta) lineages of SARS-CoV-2 as variants of concern and a further five lineages, including B.1.621 (mu), as variants of interest.
Although delta is currently responsible for the majority of infections globally, the emergence of mu has caused significant concern following reports of its higher propensity for escape from infection- or vaccine-induced immunity.
What did the researchers do?
Miyakawa and colleagues investigated the efficacy of Pfizer-BioNTech’s BNT162b2 vaccine in generating neutralizing antibodies against the original D614G variant and the mu, alpha, beta, gamma, delta, and lambda lineages. They also tested the neutralizing efficacy of the casirivimab and imdevimab monoclonal antibody cocktail against these variants.
The team performed a rapid virus-like particle-based rapid neutralization test on sera collected from individuals one week after receiving a second dose of BNT162b2 and then graded the efficacy of the neutralizing antibody response against the different variants.
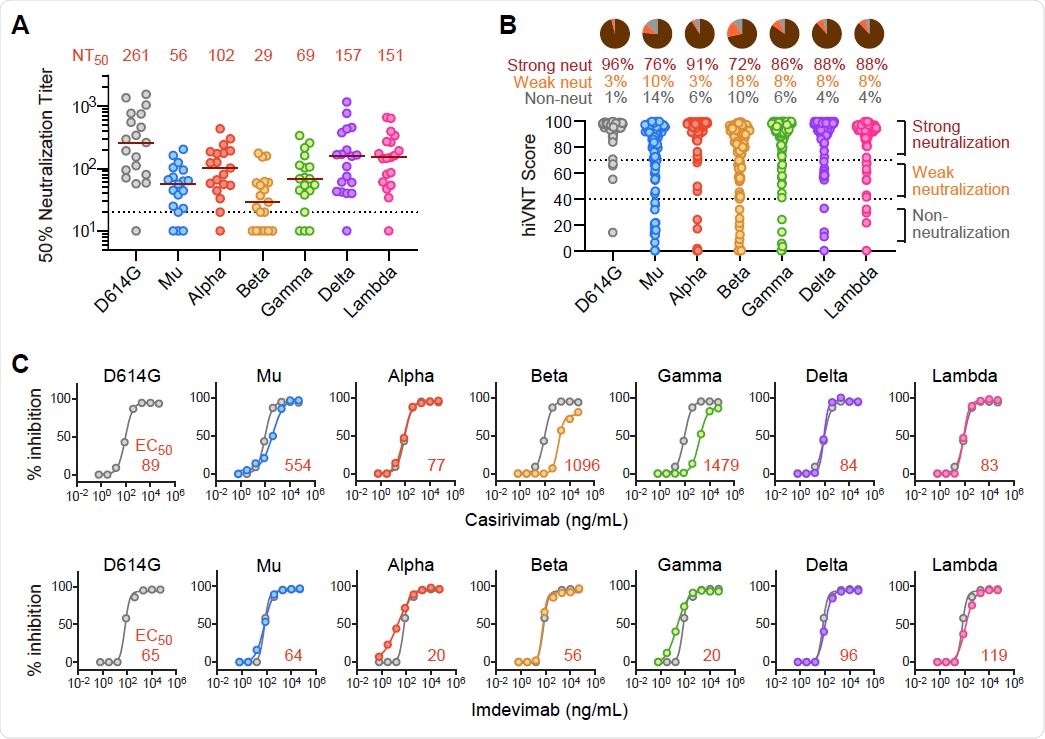
Neutralization of SARS-CoV-2 Mu variant by vaccine sera and monoclonal antibodies (A) Neutralizing activity of BNT162b2 post-vaccination sera (1 week after second dose) against each variant (n=19). Serum dilutions showing 50% inhibition of infection (NT50) were determined by quantitative hiVNT. The dotted line indicates the cut-off threshold of this assay (NT50=20). The red lines indicate the median NT50 and the values of which are displayed as red numerals above each parameter in the graph. Note that this assay correlates well with the assay using the authentic virus (see Figure S1A). (B) Positive rates for neutralizing antibody determined by qualitative hiVNT. BNT162b2 post-vaccination sera (1 week after second dose) were used (n=105). The percentage of inhibition of viral infection by 20-fold dilution of serum is shown in the scatter plot as hiVNT score. hiVNT score below 40 (equivalent to pvNT50 < 50) is defined as nAb-negative serum, 40-70 (equivalent to pvNT50 > 50 but < 200) as weakly neutralizing serum, and above 70 (equivalent to pvNT50 > 200) as strongly neutralizing serum. The percentage of each serum is shown in the pie chart. Note that the qualitative hiVNT provides semi-quantitative results (see Figure S2B for the description of the accuracy of this definition). (C) Neutralization curve of each mutant strain by two monoclonal antibodies (Casirivimab and Imdevimab). The red numbers indicate the 50% effective concentration (EC50, ng/mL). For comparison, the neutralization curve for each mutant is superimposed on that of the D614G control (gray line). Since these nAb medicines are treated as a cocktail, they are considered effective if the EC50 of either antibody is equivalent to or lower than that of the D614G control.
What did the study find?
The median neutralization efficacy against all variants was above the effectiveness threshold for all sera tested, suggesting that the vaccine-induced antibodies can neutralize most variants, including mu.
The proportion of samples that were strongly neutralized was highest for those containing the D614G variant (96%) and lowest for those containing the beta variant (72%). For the mu lineage, substantial neutralization was observed in 76% of samples.
The highest occurrence of neutralizing antibody escape (including weak and non-neutralizing activity) was observed for the beta variant (28%), followed by the mu variant (24%).
The team also found that all of the variants tested were effectively neutralized by at least one of casirivimab and imdevimab contained in the antibody cocktail.
Finally, the study found that mu caused cell-to-cell fusion, which the researchers say is highly likely to promote resistance to vaccine-induced neutralizing antibodies.
What are the implications of the study?
Miyakawa and colleagues say the results show that both the vaccine-induced neutralizing antibodies and the monoclonal antibody cocktail still possess adequate neutralization efficacy against the mu variant.
However, the researchers warn that since neutralizing antibodies can wane over time, follow-up studies are needed to assess the duration of neutralizing activity against this variant.
Furthermore, mu possessing the property of cell fusion could become a problem if this variant were to replace delta as the most predominant lineage, they add.
“Despite this, the current vaccines and antibody cocktail would still work for the majority, albeit at a slightly lowered efficacy than currently observed with the delta variant,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.