A team of scientists from China and the USA has recently described the antiviral efficacy of a monoclonal antibody candidate targeting the spike receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Importantly, they have observed that emerging spike mutations could not affect the potency of this antibody. The study is currently available on the bioRxiv* preprint server.
The most effective way to control the coronavirus disease 2019 (COVID-19) pandemic is mass vaccination together with the implementation of non-pharmaceutical control measures (mask-wearing, social distancing, etc.).
Apart from vaccines, many therapeutic monoclonal antibodies have been developed to neutralize the deadly SARS-CoV-2. The majority of these antibodies have been developed against the spike RBD as it is the most immunogenic viral component. This highlights the possibility that emerging viral variants with multiple spike mutations may affect the antiviral efficacy of these antibodies.
In this context, studies have shown that the delta variant of SARS-CoV-2, which was first identified in India, is highly resistant to neutralization by antibodies that target RBD or non-RBD epitopes.
The study
In the current study, the scientists have evaluated the antiviral efficacy of a panel of neutralizing monoclonal antibodies targeting the spike RBD of SARS-CoV-2.
They have isolated RBD+ single B cells from COVID-19 recovered individuals and cloned monoclonal antibodies. After several rounds of extensive screening, they have identified a panel of antibodies with high virus-neutralizing efficacy. Of these antibodies, one, designated as 2G1, has shown the highest neutralizing ability against all variants of concern (VOCs), including the alpha, beta, gamma, and delta variants.
Given these findings, they have specifically characterized the structural, biological, and clinical properties of the 2G1 monoclonal antibody.
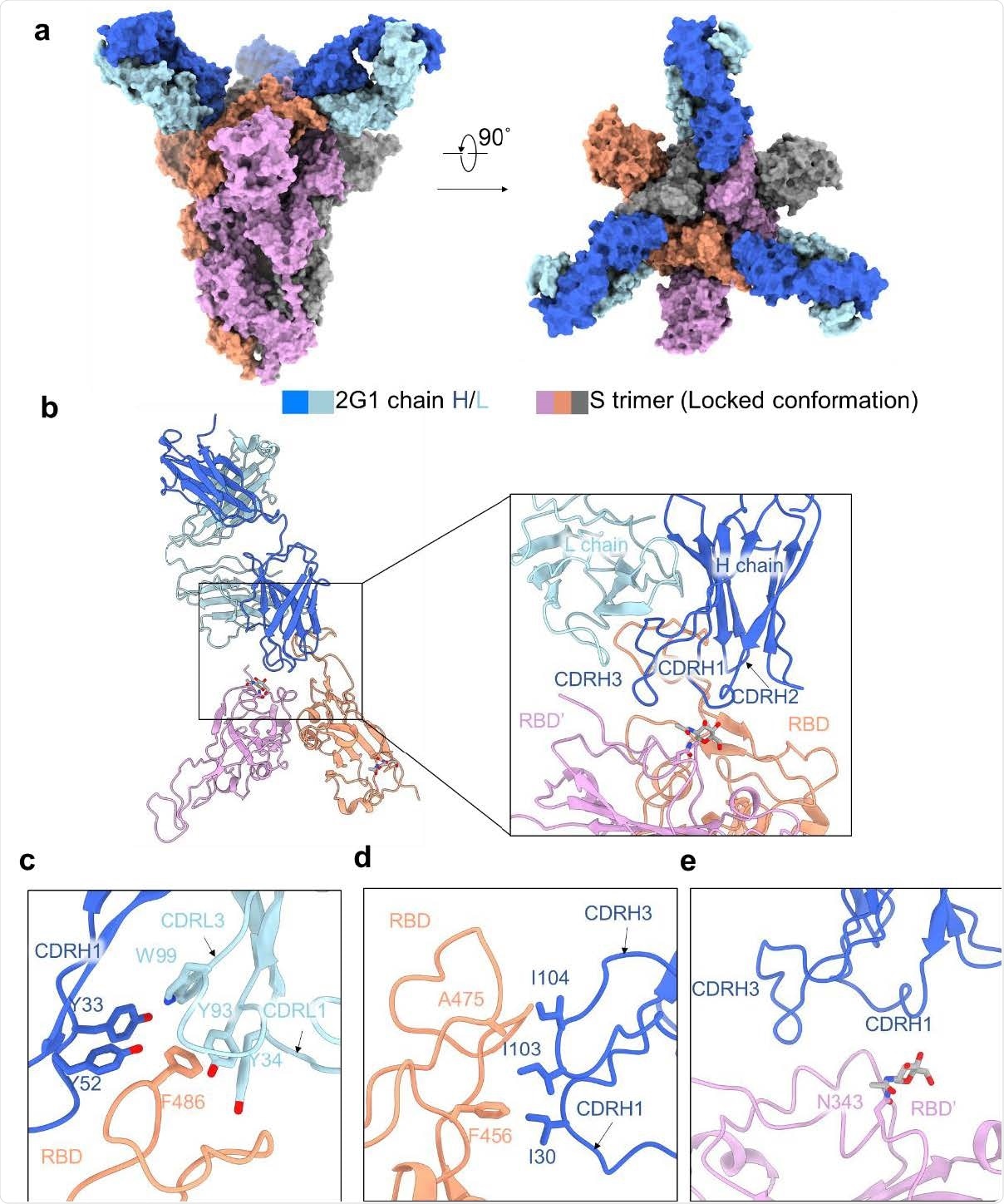
Cryo-EM structure of 2G1 and the complex with WA1/2020 S protein. a, The domain-colored cryo-EM map of SARS-CoV-2 S ectodomain trimer and 2G1 Fab fragments complex is shown, viewed along two perpendicular orientations. The heavy and light chains of 2G1 are colored blue and cyan, respectively. b, The three protomer of trimeric S protein are colored grey, orange and pink. c-e, The binding interface between 2G1 and RBD and adjacent RBD’. RBD and 2G1 interact each other mainly through hydrophobic interactions (c and d). 2G1 heavy chain (CDRH3 and CDRH1) lie above the adjacent RBD’ (e)
Structural analysis of 2G1 – spike complex
The scientists conducted a Cryo-electron microscopic analysis of the 2G1 – spike structure. The findings revealed that the antibody binds to the tip area of RBD that partially overlaps with the angiotensin-converting enzyme 2 (ACE2) binding site. Moreover, the findings showed that the small contact interface between 2G1 and RBD tip is highly stabilized by a strong hydrophobic interaction network.
To investigate the impact of spike mutations on 2G1 efficacy, the scientists mutated a series of spike residues near the antibody epitope. The findings revealed that while 484K, 477N/484Q/490S, and 477R/478K/484K mutations significantly reduce the antibody binding, 477N/490S, 477R/490S, 478K/484Q, and 484K/490S remarkably enhance it.
Furthermore, only a moderate reduction in antibody binding ability was observed by mutating major interacting residues between RBD and 2G1. Overall, these observations indicate that 2G1 is highly resistant to spike mutations and can be used as a therapeutic intervention against future SARS-CoV-2 variants.
Neutralizing ability of 2G1
The scientists conducted ACE2 – RBD interaction blocking assays to determine the binding affinity of 2G1 for several RBD mutants present in VOCs. The findings revealed that 2G1 could effectively bind to wild-type SARS-CoV-2 and its variants alpha, beta, gamma, kappa, and delta at nanomolar concentrations.
Moreover, the antibody exhibited a robust ability to block the interaction of ACE2 with spike proteins of alpha, beta, gamma, and delta variants. The overlap between antibody epitope and ACE2 binding site could be responsible for such robust ACE2 blocking ability.
The findings of live virus neutralization assays revealed that 2G1 neutralizes both wild-type SARS-CoV-2 and the delta variant with similar efficacy. Importantly, the antibody exhibited 2-fold, 5-fold, and 3-fold higher neutralization efficacy against the alpha, beta, and gamma variants, respectively, compared to that against the wild-type virus. Overall, the antibody showed neutralizing efficacy at sub-nanomolar concentrations.
Protective efficacy of 2G1
To evaluate the antiviral efficacy of 2G1 in vivo, the scientists challenged human ACE2-expressing mice with wild-type SARS-CoV-2, beta variant, or delta variant, followed by treatment with three different doses (20, 6.7, and 2.2 mg/kg body weight) of the antibody.
The findings revealed that wild-type SARS-CoV-2-infected or beta-infected mice that were treated with different antibody doses survived without showing any disease-related symptoms. Similar protection was observed in delta-infected mice that were treated with the highest antibody dose.
To test the antiviral efficacy of 2G1 in non-human primates, the scientists infected monkeys with wild-type SARS-CoV-2 and treated them with 10 mg/kg or 50 mg/kg of the antibody. In all treated monkeys, a complete clearance of the virus from the throat was observed at day 4 post-infection. Moreover, no fecal shedding of the virus was observed.
The toxicity analysis revealed that the antibody is safe and well-tolerated in animals, even at very high concentrations. Moreover, the antibody did not exhibit any antibody-dependent cellular cytotoxicity effect.
Study significance
The study identifies a promising monoclonal antibody candidate that has high neutralizing potency against SARS-CoV-2 VOCs. Given the observed protective efficacy in animals, the scientists believe that the antibody could clinically benefit COVID-19 patients.
Source
Ma H. 2021. Broad ultra-potent neutralization of SARS-CoV-2 variants by monoclonal antibodies specific to the tip of RBD, BioRxiv, https://www.biorxiv.org/content/10.1101/2021.09.24.461616v1

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ma H. 2021. Broad ultra-potent neutralization of SARS-CoV-2 variants by monoclonal antibodies specific to the tip of RBD, BioRxiv, https://www.biorxiv.org/content/10.1101/2021.09.24.461616v1
- Peer reviewed and published scientific report.
Ma, Hang, Yingying Guo, Haoneng Tang, Chien-Te K. Tseng, Lei Wang, Huifang Zong, Zhenyu Wang, et al. 2022. “Broad Ultra-Potent Neutralization of SARS-CoV-2 Variants by Monoclonal Antibodies Specific to the Tip of RBD.” Cell Discovery 8 (1): 1–14. https://doi.org/10.1038/s41421-022-00381-7. https://www.nature.com/articles/s41421-022-00381-7.