It has been demonstrated in recent studies that there is a significant breadth of emerging autoreactivity associated with severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. More specifically, researchers from Emory University have identified a relaxation of peripheral tolerance within early antibody secretion cells that arise in individuals infected with SARS-CoV-2, as important drivers of those responses.
These extrafollicular-derived cells are often virus-specific but also show cross-reactivity to autoantigens located in the inflammatory lung environment, including naïve B cell reactivity and glomerular basement membrane.
Within some patients, despite most autoreactivity being resolved within six months, anti-nuclear antibodies (ANA) and anti-carbamylated protein (CarP) responses were highly represented. Questions were raised from these results by the authors regarding autoreactive antibodies that arise during acute SARS-CoV-2 infections and their persistence in patients with symptoms in Post-Acute Sequelae of SARS-CoV-2 infection (PASC).
A preprint version of this study, which is yet to undergo peer review, is available on the medRxiv* server.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
What did the authors do?
To examine in depth the chance that autoreactivity is over-represented in individuals diagnosed with PASC, 95 patients with differing symptoms of COVID-19 were progressively recruited upon arrival to two academic PASC clinics in Atlanta, Georgia, USA, during the periods of December 2020-June 2021. The participants had a median age of 50 years old (range 21-81), 76% were female, and the majority were African American (58%). Most of the participants were displaying mild acute COVID-19, while 40 of the participants required hospitalization.
When the samples were taken from each patient, they were a median of 110 days from the onset of COVID-19 symptoms. Out of all the PASC symptoms reported by the patients, the most common were dyspnea (69%), general fatigue (64%), and brain fog (47%). Excluded from the cohort were patients who have been previously diagnosed or who were suspected of having a rheumatic disease prior to the diagnosis of COVID19.
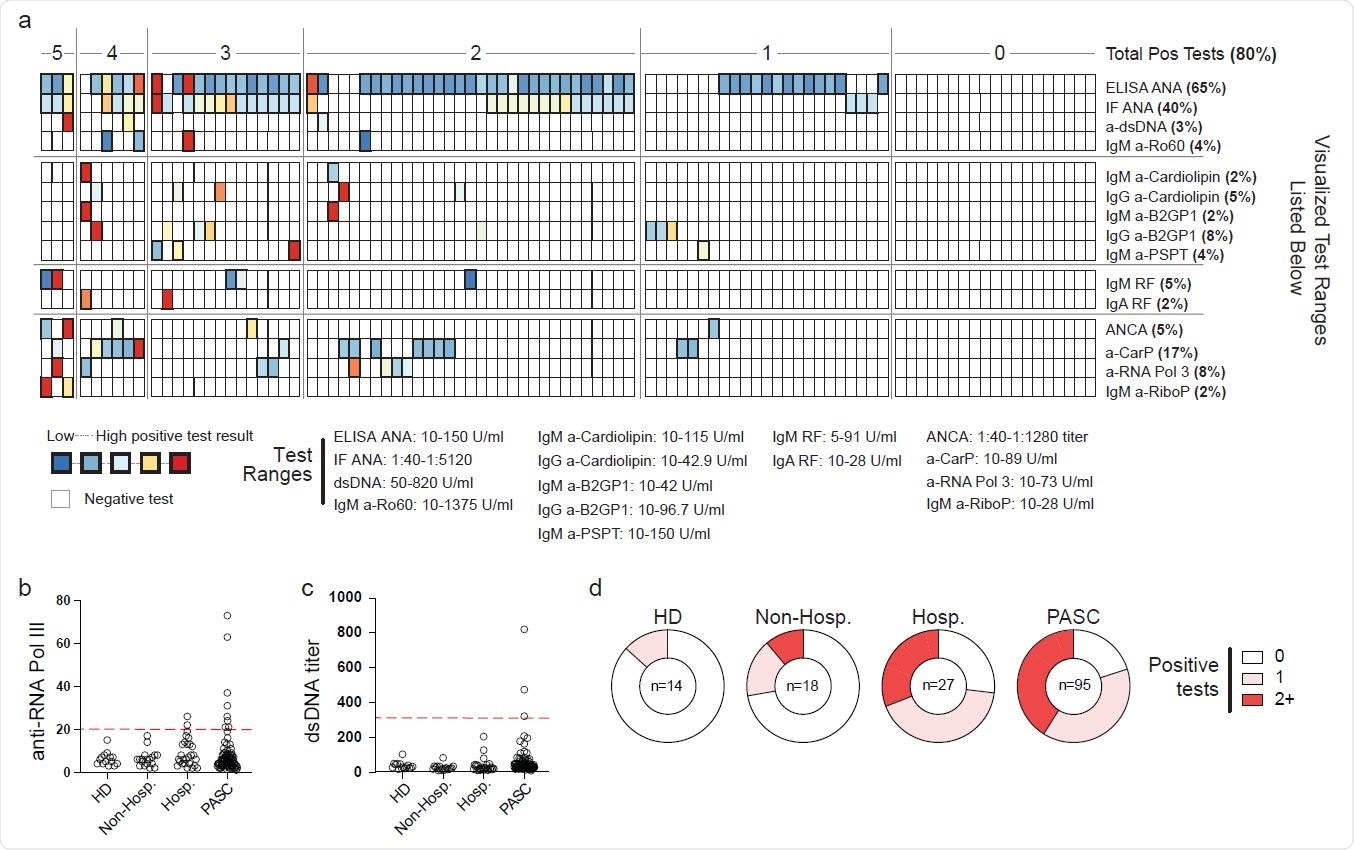
Autoreactivity in Post-Acute Sequelae of SARS-CoV-2 Plasma from 95 patients with mixed PACS symptomology was screened by Exagen clinical laboratory for reactivity against 30 clinically-relevant autoantigens. (a) Heatmap of patient results. Each column represents a single patient grouped by the total number of autoreactive positive tests that the patient displayed. Bolded boxes represent clinical positive tests with the color indicating the magnitude of the test result. Scale for each test is documented below the heatmap. (b-c) Autoantibody titers from heathy donor (HD), acute mild/moderate (mild/mod) COVID-19, acute severe/critical (sev/crit) COVID-19, or PASC patient groups. Red line indicates clinical positive test threshold. (b) Anti-RNA polymerase III (RNA Pol III) titers. (c) Anti-double stranded DNA (dsDNA) titers. (d) Frequency of the total number of positive autoantigen tests from indicated patient groups.
What did the authors discover?
Consistent with the patients hospitalized with COVID-19, 80% of PASC patients produced positive tests for reactivity against at least one autoantigen, with increased ANAs (63%) specifically common among the participants. Also, among the cohort who returned positive tests, 16% and 8% of patients had elevated anti-CarP responses alongside reactivity against RNA polymerase 3.
Interestingly, double-stranded DNA was uniquely increased within the PASC patients, with 3 patients returning positive clinical tests and 4 more that were expressing titers exceeding 5 standard deviations of a matched samples from healthy individuals. It was common for the authors to observe multiple breaks in tolerance, with 40% of patients showing tolerance breaks against two or more independent autoantigens.
Implications
The authors displayed evidence of serum autoantibodies in patients who attended PASC clinics with persistent symptoms lasting up to 14 months following a SARS-CoV-2 infection. It will require further study to determine whether these autoantibodies in patients early post-recovery with PASC have a relationship with long-term persistence of symptoms and eventual diagnosis of autoimmune disease. The data presented in this paper confirm the link between COVID-19 and observed clinical autoreactivity, even when individuals are in the recovery phase of the disease.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Evidence of Persisting Autoreactivity in Post-Acute Sequelae of SARS-CoV-2 Infection Matthew Woodruff, Tiffany Walker, Alexander Truong, Jenny Han, Adviteeya Dixit, Richard Ramonell, Martin Runnstrom, Mark Rudoloph, Arezou Khosroshahi, F. Eun-Hyung Lee, Ignacio Sanz, medRxiv, 2021.09.21.21263845; doi: https://doi.org/10.1101/2021.09.21.21263845, https://www.medrxiv.org/content/10.1101/2021.09.21.21263845v1