Is one COVID vaccine better than the other? Four current vaccines — Moderna, Pfizer-BioNTech, Johnson & Johnson, and AstraZeneca — are highly effective against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but a new medRxiv* study suggests the level of protection varies.
Researchers from The Netherlands found the mRNA vaccines produce more antibodies than adenovirus-based vaccines.
“The implication is that individuals receiving one of the adenovirus vector-based vaccines are more vulnerable to infection with the Beta and Delta VOCs, which is consistent with the lower efficacy of these vaccines against symptomatic infection with VOCs compared to the mRNA vaccines, although all vaccines are highly effective at preventing severe disease by VOCs,” explained the researchers.
Against SARS-CoV-2 variants, especially the Beta variant, immunity weakened most among adenovirus vector-based vaccines.
Overview of experiment
The researchers collected convalescent plasma samples of individuals who were vaccinated with either the Pfizer-BioNTech (n = 50), Moderna (n = 40), AstraZeneca (n = 41), or the single-shot Johnson & Johnson vaccine (n = 16).
Participants included healthcare workers who were never exposed to SARS-CoV-2. The four vaccine groups were fairly similar in composition; 62-87% were female, with the majority between 35 and 60 years old. However, the majority of individuals who were vaccinated with the two-dose AstraZeneca shot were over 60 years of age. The age difference is due to the Dutch government allocating most AstraZeneca shots to this age group.
Neutralizing antibodies were measured from serum samples and tested against four SARS-CoV-2 variants of concern — Alpha, Beta, Gamma, and Delta.
Serum samples were collected at least 3 weeks after the first vaccination and 4 weeks after the second vaccination. For the one-dose Johnson & Johnson shot, samples were collected 5 to 8 after vaccination.
Both mRNA vaccines show superior humoral immune response
All four vaccines showed antibody responses against the spike protein of the original coronavirus strain.
Four weeks after full dosing, mRNA vaccines had significantly higher neutralization titers compared to adenovirus-based vaccines. However, antibody responses decreased in individuals who received the AstraZeneca and the Johnson & Johnson vaccine.
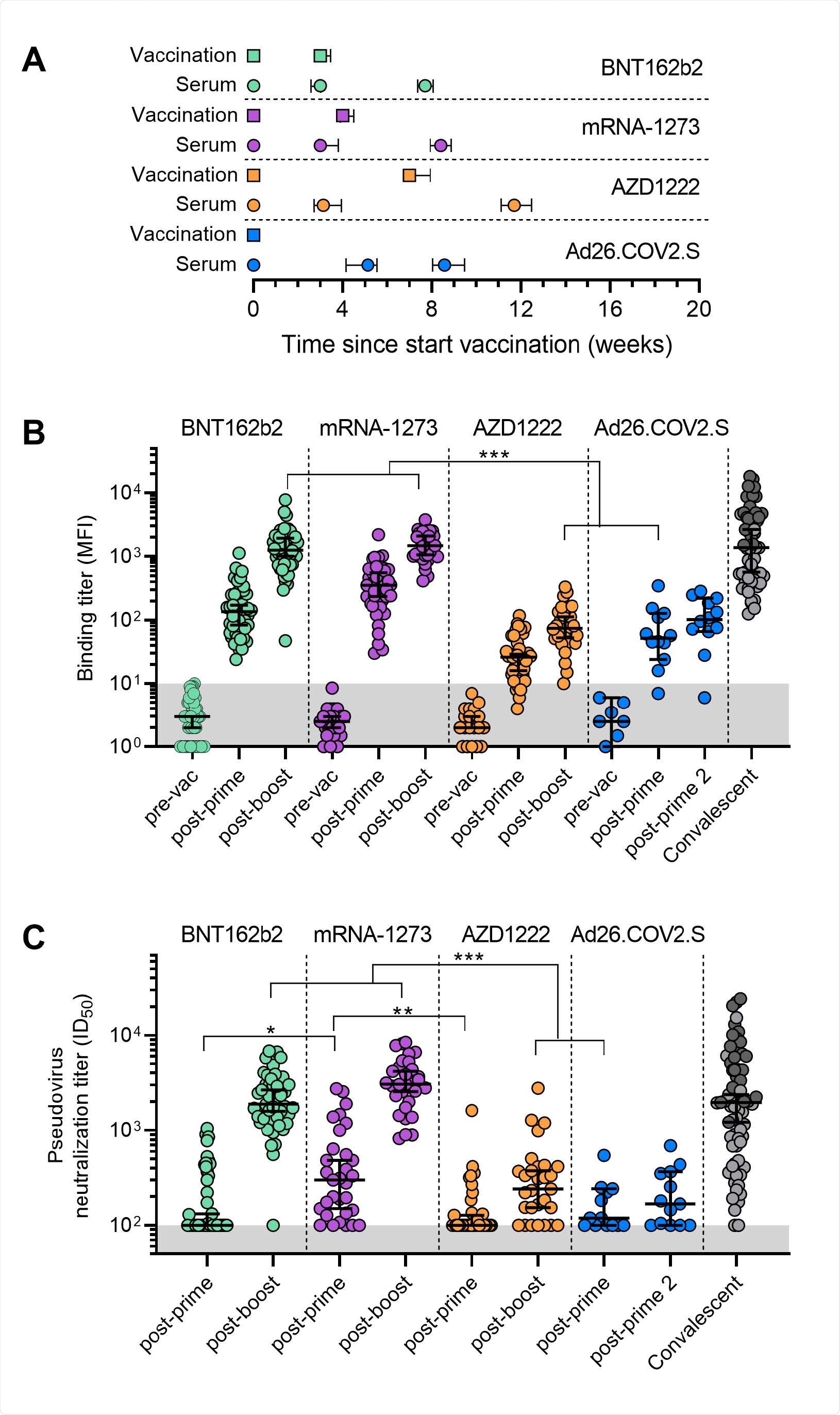
Binding and neutralization titers pre- and post-vaccination with one of the four SARS-CoV-2 vaccines. (A) Timelines of the vaccinations and serum collections, showing the mean and interquartile range of times of vaccination and samples in weeks after the first dose. (B) Binding titers to wild-type S protein represented as mean fluorescence intensity (MFI) of 1:100,000 diluted sera collected pre- and post-vaccination for the four vaccination groups. The convalescent group (n=68) consists of sera from hospitalized (dark gray) and non-hospitalized (light gray) COVID-19 patients collected 4-6 weeks post symptom onset. Median and 95% confidence intervals are indicated. The lower cutoff for binding was set at an MFI of 10 (grey shading). (C) Median half-maximal neutralization (ID50) titers of D614G pseudovirus for sera collected post-vaccination for the four vaccination groups. The convalescent group (n=68) consists of sera from hospitalized (dark gray) and non-hospitalized (light gray) COVID-19 patients collected 4-6 weeks post symptom onset. Median and 95% confidence intervals are indicated. The lower cutoff for neutralization was set at an ID50 of 100 (grey shading). All data points shown here represent the mean of a technical triplicate. *, p < 0.01, **, p < 0.001, ***, p < 0.0001.
The AstraZeneca and the Johnson & Johnson vaccine had a 17 to 29-fold reduction in antibody responses.
Using a lentiviral-based pseudovirus, the researchers studied vaccine antibody responses against SARS-CoV-2 variants.
For the B.1 variant, neutralizing antibody responses were highest with the Pfizer-BioNTech and the Moderna vaccines. But the Johnson & Johnson and AstraZeneca vaccines had lower neutralizing activity against the variant.
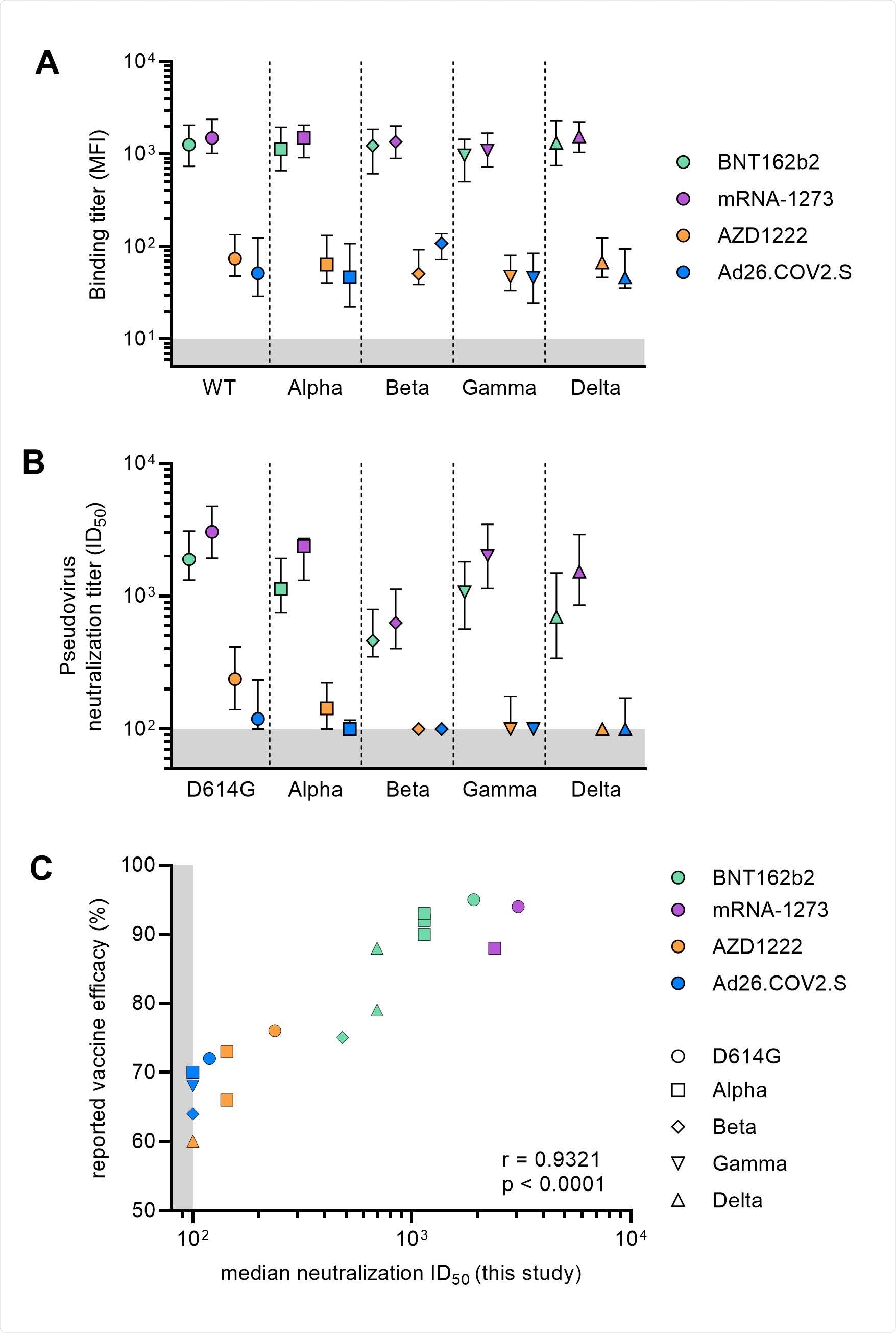
Binding and neutralization titers post-vaccination against VOCs. (A) Median with an interquartile range of binding titers to wild-type and VOCs S proteins represented as mean fluorescence intensity (MFI) of 1:100,000 diluted sera collected four-five weeks after full vaccination for the four vaccination groups. The lower cutoff for binding was set at an MFI of 10 (grey shading). Vaccine groups are indicated by colors with BNT162b2 in green, mRNA-1273 in purple, AZD1222 in orange and Ad26.COV2.S in blue. (B) Median with an interquartile range of half-maximal neutralization (ID50) titers of D614G and VOCs pseudoviruses for sera collected after full vaccination for the four vaccination groups. The lower cutoff for neutralization was set at an ID50 of 100 (grey shading). Vaccine groups are indicated by colors with BNT162b2 in green, mRNA-1273 in purple, AZD1222 in orange and Ad26.COV2.S in blue. (C) Median ID50 neutralization of D614G and VOCs plotted against the reported vaccine efficacy against symptomatic infection. Vaccine groups are indicated by colors with BNT162b2 in green, mRNA-1273 in purple, AZD1222 in orange and Ad26.COV2.S in blue. Circles represent WT data, squares for Alpha, diamond for Beta, nabla triangle for Gamma and delta triangle for Delta. Spearman's rank correlation coefficient with p-value are indicated. The result of the AZD1222 phase 3 trial conducted in South Africa, demonstrating poor (10%) efficacy against Beta variant, is not shown.
Antibody response after partial vaccination
The researchers next look at antibody responses after one vaccination compared to the single-dose Johnson & Johnson vaccine.
The mRNA vaccines had detectable antibody binding titers against the spike protein after one dose. In contrast, 5 of the 42 individuals taking one-dose AstraZeneca vaccine and 1 of the 13 individuals with the Johnson & Johnson vaccine did not show antibody levels.
A one-dose Moderna vaccine had the highest antibody titers compared to all three vaccines.
Overall, partial vaccination resulted in low neutralizing antibody levels than full vaccination. “The neutralizing antibody levels after one dose were low in all cases (median titers of <100 for BNT162b2 and AZD1222, 119 for Ad26.COV2.S and 300 for mRNA-1273) with only 19 of 45 (42%) BNT162b2, 13 of 35 (37%) AZD1222, 7 of 13 (54%) Ad26.COV2.S and 26 of 31 (84%) mRNA-1273 having detectable neutralization,” explained the researchers.
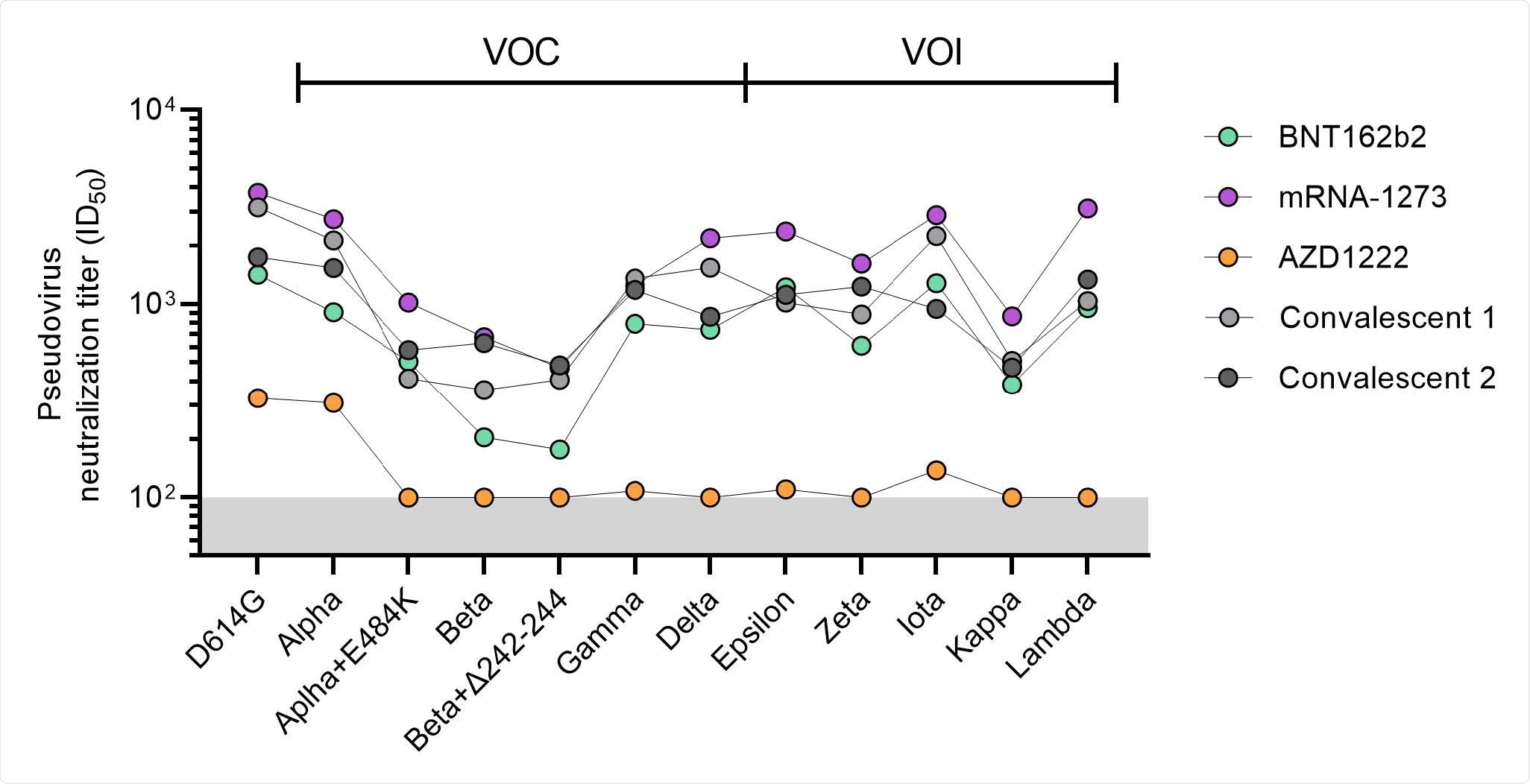
Neutralization titers of pooled sera against VOCs and VOIs. Half-maximal neutralization (ID50) titers of SARS-CoV-2 variant pseudoviruses for pooled sera for the vaccination groups (excluding the Ad26.COV2.S group) after full vaccination. The lower cutoff for neutralization was set at an ID50 of 100 (grey shading). Convalescent group 1 (light gray) consists of pooled COSCA sera representing COVID-19 patients between 4-6 weeks post symptom onset and convalescent group 2 (dark gray) consists of pooled RECoVERED sera representing COVID-19 patients up to seven months post symptom onset (median three months), who experienced mild to severe COVID-19. Vaccine groups are indicated by colors with BNT162b2 in green, mRNA-1273 in purple and AZD1222 in orange. All data points shown here represent the mean of a technical triplicate and at least two replications.
Antibody levels against variants of concern
All vaccines showed a drop in antibody levels against SARS-CoV-2 variants, with the most significant reduction in neutralizing activity coming from the Beta variant, followed by Delta, Gamma, and Alpha.
Neutralizing titers against the Alpha, Beta, Gamma, and Delta variants were the highest among individuals with either of the mRNA vaccines.
A good proportion of recipients of the AstraZeneca and Johnson & Johnson vaccines did not show detectable neutralizing activity against SARS-CoV-2 variants.
Neutralization titers were also associated with greater protection from symptomatic COVID-19 infection.
“Overall, the results show that the mRNA vaccines induce substantial levels of neutralizing antibodies against currently defined VOCs, while the adenovirus vector-based vaccines are much less efficient in doing so,” concluded the researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Van Gils MJ, et al. (2021). Four SARS-CoV-2 vaccines induce quantitatively different antibody responses against SARS-CoV-2 variants. medRxiv, 2021. Doi: https://doi.org/10.1101/2021.09.27.21264163, https://www.medrxiv.org/content/10.1101/2021.09.27.21264163v1
- Peer reviewed and published scientific report.
Gils, Marit J. van, Ayesha Lavell, Karlijn van der Straten, Brent Appelman, Ilja Bontjer, Meliawati Poniman, Judith A. Burger, et al. 2022. “Antibody Responses against SARS-CoV-2 Variants Induced by Four Different SARS-CoV-2 Vaccines in Health Care Workers in the Netherlands: A Prospective Cohort Study.” Edited by James G. Beeson. PLOS Medicine 19 (5): e1003991. https://doi.org/10.1371/journal.pmed.1003991. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003991.