Researchers in the United States have demonstrated the real-world effectiveness of Moderna’s mRNA-1273 vaccine at protecting against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and preventing hospitalization due to coronavirus disease 2019 (COVID-19).
The team – from Kaiser Permanente Southern California in Pasadena and Moderna Inc. in Cambridge, Massachusetts – found that double vaccination with mRNA-1273 was protective against infection with multiple SARS-CoV-2 variants, including the B.1.617.2 (delta) lineage that has become the dominant strain worldwide.
Katia Bruxvoort and colleagues report that the vaccine efficacy against infection with delta was moderately lower than against non-delta variants, at 86.7% versus 90.4 to 98.4%. However, the efficacy against hospitalization following delta infection was as high as 97.6%.
Bruxvoort and colleagues also warn that efficacy against delta infection declined with increasing time since vaccination and that further research is required to inform booster dose strategies.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Concerns about the effectiveness of vaccination against delta variant
Since the COVID-19 pandemic began in late December 2019, several effective vaccines have rapidly been developed to protect against infection with the causative agent SARS-CoV-2.
In clinical trials, the messenger RNA- (mRNA) based vaccines developed by Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2) were shown to be highly effective (94% and 95%, respectively) at protecting against symptomatic COVID-19.
After these vaccines received emergency use authorization in the United States in December 2020, mass immunization programs were deployed in both high-risk and general populations.
Multiple real-world studies subsequently reported that mRNA-based vaccination was highly effective at protecting against SARS-CoV-2 infection (82 to 100%) and COVID-19 hospitalization (87 to 96%).
However, these studies were conducted before the delta lineage became predominant and concerns have arisen that the vaccines may be less effective against this variant.
The increased transmissibility of delta led to a surge in infections, hospitalizations, and deaths across the United States, and some studies have reported decreased vaccine efficacy (51 to 75%) against infection with this variant.
However, it is not clear whether these findings are due to a lower protective effect against delta, waning of vaccine-induced immunity over time or other factors. Furthermore, few studies have specifically examined the efficacy of mRNA-1273 against delta or other SARS-CoV-2 variants.
“Such studies are critically needed to inform ongoing decisions around booster doses and development of vaccines that may offer broad protection against SARS-CoV2 variants,” says Bruxvoort and colleagues.
What did the researchers do?
Now, the researchers have evaluated the efficacy of the mRNA-1273 vaccine over time in a study conducted at Kaiser Permanente Southern California –an integrated health care system covering 15 hospitals across Southern California.
The team performed whole-genome sequencing of SARS-CoV-2-positive samples collected between March 1st and July 27th, 2021. Test-positive cases were matched to test-negative controls based on age, sex, ethnicity and date of sample collection.
What did the study find?
The study included 8,153 test-positive cases, of which variants were identified for 5,186 (63.6%).
Among the variants identified, 39.4% were delta, 27.7% were B.1.1.7 (alpha), 11.4% were B.1.427/429 (epsilon), 6.9% were P.1 (gamma), 2.2% were B.1.526 (iota), 1.4% were B.1.621 (mu), and 11.1% were other lineages.
Among fully vaccinated cases (those who had received two doses), 85.0% of the variants identified were of the delta lineage.
Full vaccination was found to be 86.7% protective against infection with delta, which was moderately lower than the efficacy observed for alpha (98.4%). Efficacy was 90.4% against mu and ranged from 95.5 to 97.6% for other non-delta variants.
The efficacy of one vaccine dose was lower against all variants, ranging from 45.8% against mu to 90.1% against alpha.
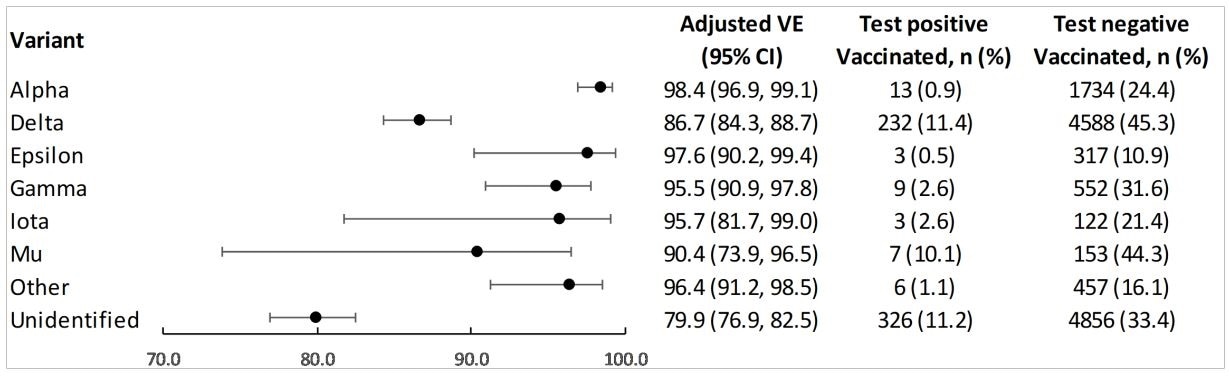
Vaccine effectiveness of 2 doses of mRNA-1273 against infection with SARS-CoV-2 variants
Efficacy waned with increasing time since vaccination
Efficacy against delta was highest (94.1%) 14 to 60 days after receiving the second dose but declined moderately to 80.0% at 151 to 180 days after the second dose.
Waning efficacy was less pronounced for non-delta variants, declining from 97.3 to 99.3% at 14 to 60 days to 73.2 to 95.2% at 121 to 150 days.
Efficacy against delta infection was lower among individuals aged 65 years or older than among those aged 18 to 64 years, at 75.2% versus 87.9%.
Among those aged 18 to 64 years, efficacy against delta fell from 95.1% at 14 to 60 days following the second dose to 79.4% at 151 to 180 days.
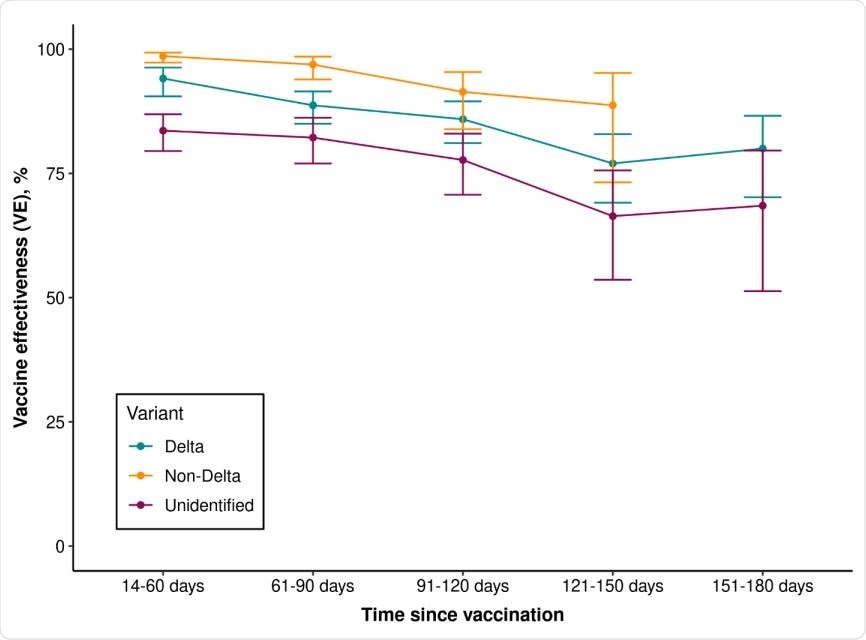
Vaccine effectiveness of 2 doses of mRNA-1273 against infection with SARS-CoV-2 variants by time since vaccination
Robust protection against hospitalization
Robust protection against hospitalization following delta infection was observed throughout the study period, with efficacy reaching as high as 77% following one vaccine dose and 97.6% following the second dose.
The researchers say this real-world study provides evidence of high vaccine efficacy following two doses of mRNA-1273 against infection with multiple SARS-CoV-2 variants, including delta.
However, the decline in efficacy over time has implications for booster doses and further research is required to inform booster strategies, says Bruxvoort and colleagues.
“Efforts to deploy booster doses must not replace efforts to reach unvaccinated individuals, who comprise most COVID-19 hospitalizations and deaths,” warns the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bruxvoort K, et al. Effectiveness of mRNA-1273 against Delta, Mu, and other emerging variants. medRxiv, 2021. doi: https://doi.org/10.1101/2021.09.29.21264199 https://www.medrxiv.org/content/10.1101/2021.09.29.21264199v1
- Peer reviewed and published scientific report.
Bruxvoort, Katia J., Lina S. Sy, Lei Qian, Bradley K. Ackerson, Yi Luo, Gina S. Lee, Yun Tian, et al. 2021. “Effectiveness of MRNA-1273 against Delta, Mu, and Other Emerging Variants of SARS-CoV-2: Test Negative Case-Control Study.” BMJ 375 (December): e068848. https://doi.org/10.1136/bmj-2021-068848. https://www.bmj.com/content/375/bmj-2021-068848.