A research group from Italy and the United States proposed a graphene field-effect transistor (gFET) biosensor that takes advantage of a critical interaction between the spike glycoprotein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its receptor on human cells – opening the door for a new generation of highly sensitive detection methods.
The need for rapid and very sensitive detection systems was evident early in the coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2; however, this issue became even more important in the era of an increased number of viral variants.
Societies worldwide are slowly opening, but the global distribution of vaccines is not up to pace, which means the causative agent of the ongoing pandemic is far from being contained. Hence, as we advance we will need to react quickly to potential disease surges, so there will be a need for rapid test results.
Still, highly sensitive molecular tests based on polymerase chain reaction (PCR) require several hours for the results and specialized personnel and adequate laboratory facilities. On the other hand, rapid antigen-based tests have much lower accuracy, which can be additionally impacted by emerging SARS-CoV-2 variants.
There is an additional issue of environmental impact, as both aforementioned testing strategies use a substantial amount of disposable plastic supplies in testing campaigns on a global level. Is there a possibility to address this issue with a highly sustainable material?
Towards an easily detectable signal
A new study posted to the medRxiv* server and led by Dr. Mattia D'Agostino, Dr. Eleonora Pavoni and Dr. Alice Romagnoli from the Polytechnic University of Marche in Ancona (Italy), a gFET biosensor that uses angiotensin-converting enzyme 2 (ACE2) as a bioreceptor has been put forth as a viable solution.
More specifically, their approach aims to mimic the viral mechanism of host cell access while the viral spike glycoprotein recognizes and binds to the ACE2 receptor, a ubiquitous membrane-anchored protein.
In the proposed gFET, a graphene monolayer links the source and drain electrodes of a transistor, while graphene is functionalized with a bioreceptor that can specifically bind target molecules. As a result, such bioreceptor-target interaction can modify graphene's electronic properties, which results in an easily detectable signal.
To achieve portability, the researchers have also designed and built a reusable, customized point-of-care device that accommodates the gFET chip and gives the same readouts of the laboratory scale probe station used during sensor implementation and testing.
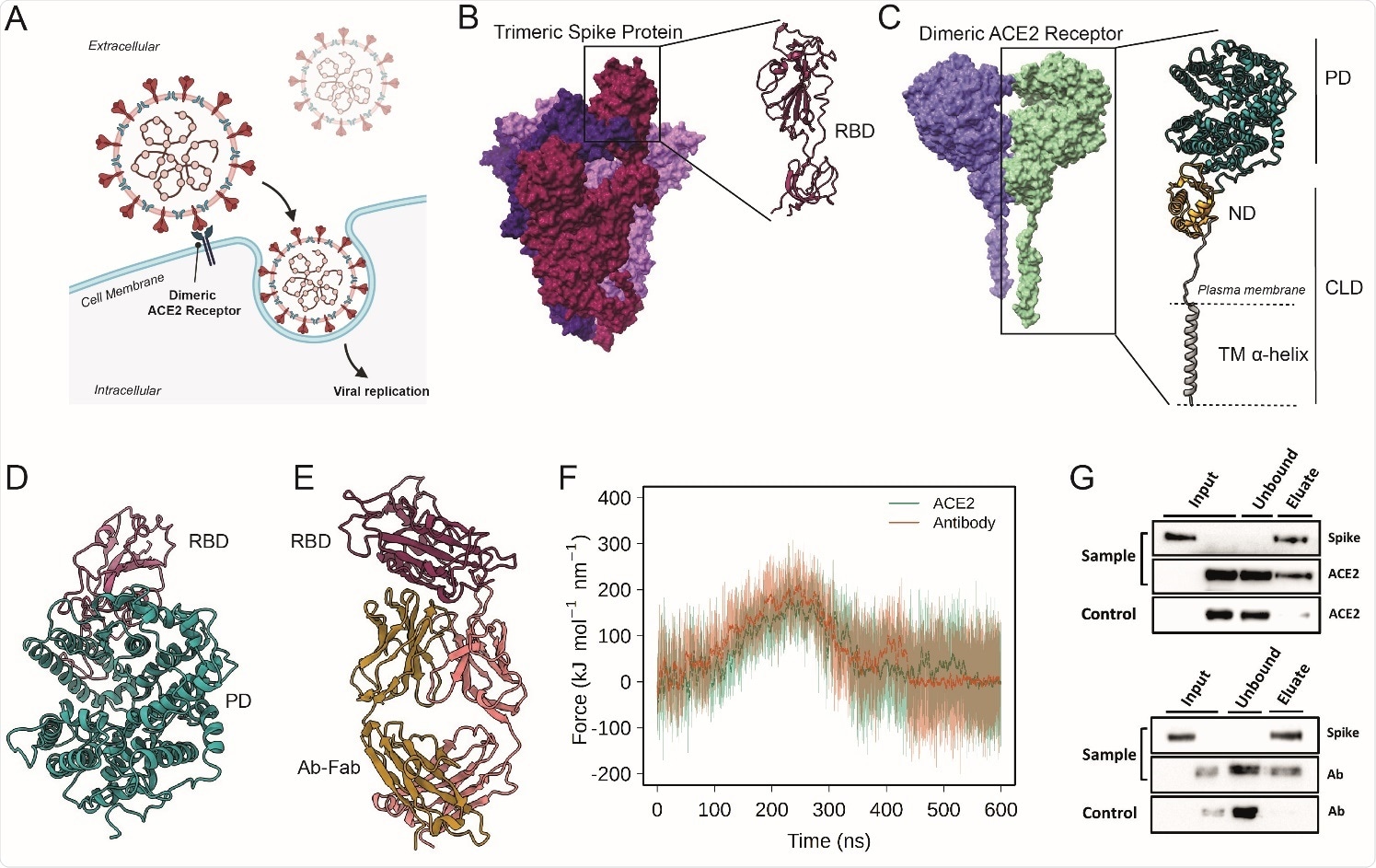
Probing the interactions of ACE2 and Antibodies with Spike (A) Schematic representation of ACE2-mediated host cell entry mechanism. (B) Cryo-EM structure of soluble trimeric Spike protein (the three monomers have different colors). A zoomed in view of the RBD is shown. (C) Cryo-EM structure of dimeric ACE2 receptor (the two monomers are colored differently). The two subunits of a monomer are reported: Peptidase domain (PD) and Collectrin-like domain (CLD) that is composed by neck domain (ND) and transmembrane helix (TM). (D) Peptidase Domain of the ACE2 receptor bound to the RBD of the SARS-CoV- 2 Spike protein. (E) Antibody CR3022, bound to the SARS-CoV-2 spike protein RBD. (F) Force profiles from the steered MD simulations of RBD unbinding from the ACE2 receptor (orange) and Ab-CR3022 (green) (G) Pull-down assay of Spike and ACE2 (upper), and Spike and Ab Anti-Spike (lower). Control is represented by the same experiment excluding the Spike protein (bait) from the system. The binding of Spike with ACE2 or anti-Spike were monitored by Western blot analysis.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Robust detection of SARS-CoV-2 variants
By using a comprehensive computational analysis approach, the researchers have shown that a chimeric ACE2-Fc construct (i.e., fusion of the fragment crystallizable part of human immunoglobulin G to the ACE2) can neatly mimic the ACE2 dimer (usually present on host cells membranes) much better than its soluble truncated form.
They have also demonstrated that ACE2-Fc functionalized gFET can be very effective for in vitro detection of SARS-CoV-2 spike glycoprotein, outperforming the same chip functionalized with either soluble ACE2 or a diagnostic antibody.
By imitating the actual interaction between the virus and the host during cell infection, whose affinity has been improved in certain variants that have emerged this year, the sensor in this study is expected to be rather robust to current and future SARS-CoV-2 variants.
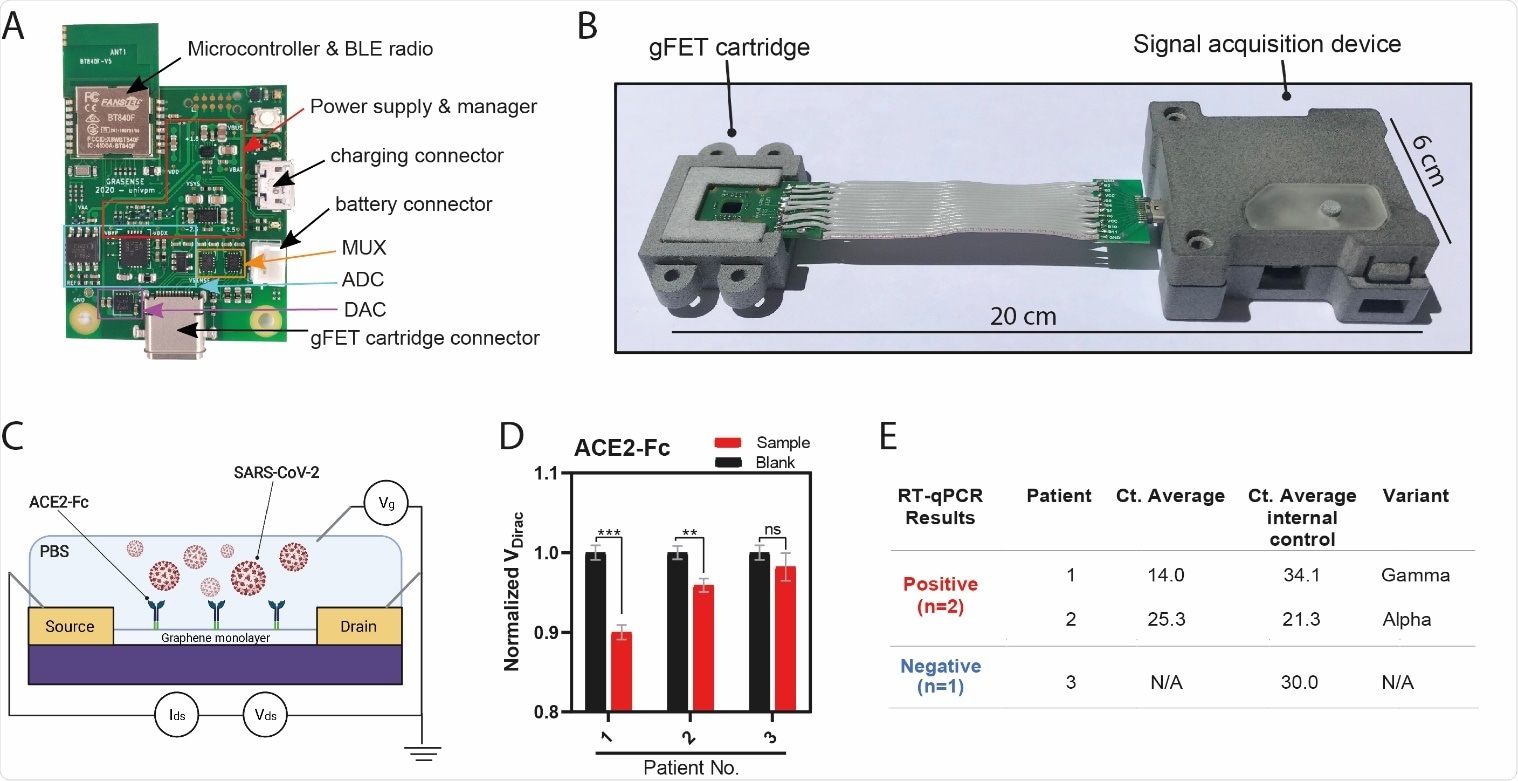
Point-of-Care (POC) device detects SARS-CoV-2 in patient samples (A) Signal acquisition module of the POC device with main elements labeled. (B) Photograph of the gFET Cartridge Unit and the Signal acquisition modules connected together to form the entire POC. A reference dimension bar is reported below. (C) Schematic representation of gFET modified with ACE2-Fc tested on viral samples from patients. (D) Bar graph reporting ACE2-Fc_gFET signal before (black) and after the addition of swab specimens (red) from patient 1, 2 and 3. **P<.01, ***P<.001; (E) RT-qPCR results for the detection of the SARS-CoV-2 specific genes (E gene; RdRP gene; S gene and ORF1ab gene) of the three patient samples. Ct value in clinical samples were evaluated. Ct average value of internal control is indicated for each sample. The type of virus variant (as detected by targeted RT-PCR) is also reported.
Low-waste and user-friendly point-of-care device
In short, gFETs seem rather attractive in point-of-care diagnosis – not only due to their miniaturization but also due to their potential for the large-scale manufacturing process and operability by non-specialized staff. These are all important facets of any diagnostic procedure in the middle of the pandemic.
"Our biosensor, miniaturized into a reusable, low-waste user-friendly point-of-care device, was successfully tested with clinical samples from patients infected by alpha and gamma virus variants," explain the authors of this medRxiv paper.
"Accuracy studies coupled with PCR-based molecular tests as a benchmark will be, of course, needed to verify the performance of our point-of-care biosensor with different and upcoming virus variants," they add.
Moreover, modifications of the ACE2 amino acid sequence that potentially increase binding affinity with the viral spike glycoprotein could additionally improve the sensitivity of the proposed ACE2-Fc gFET-based biosensor. Overall, this novel biosensor sets the stage for a new class of rapid, highly-sensitive and robust SARS-CoV-2 detection platforms, but there is a need for more research.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
D'Agostino, M. et al. (2021). SARS-CoV-2 multi-variant graphene biosensor based on engineered dimeric ACE2 receptor. medRxiv. https://doi.org/10.1101/2021.10.02.21264210, https://www.medrxiv.org/content/10.1101/2021.10.02.21264210v1
- Peer reviewed and published scientific report.
Romagnoli, Alice, Mattia D’Agostino, Eleonora Pavoni, Chiara Ardiccioni, Stefano Motta, Paolo Crippa, Giorgio Biagetti, et al. 2023. “SARS-CoV-2 Multi-Variant Rapid Detector Based on Graphene Transistor Functionalized with an Engineered Dimeric ACE2 Receptor.” Nano Today 48 (February): 101729. https://doi.org/10.1016/j.nantod.2022.101729. https://www.sciencedirect.com/science/article/pii/S1748013222003577.