The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the current coronavirus disease 2019 (COVID-19). This modern plague shut down travel, business, education, and leisure activity for months as SARS-CoV-2 quickly spread to infect hundreds of millions around the world. Achieving herd immunity has become a top priority and one which seemed to be within sight once the first vaccines were developed and found to be safe and highly effective.
However, the cost of vaccination, logistical problems, and the emergence of antibody-resistant variants of SARS-CoV-2 pose formidable challenges to this goal. An interesting new study published on the preprint medRxiv* introduces the possibility that the inactivated polio vaccine, which is among the universal early childhood vaccines, can elicit antibodies that inhibit SARS-CoV-2 nucleic acid synthesis.
 Study: Inactivated Poliovirus Vaccine Induces Antibodies that Inhibit RNA Synthesis of SARS-CoV-2: An open-label, pre-post vaccine clinical trial. Image Credit: Bernard Chantal / Shutterstock.com
Study: Inactivated Poliovirus Vaccine Induces Antibodies that Inhibit RNA Synthesis of SARS-CoV-2: An open-label, pre-post vaccine clinical trial. Image Credit: Bernard Chantal / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The first four COVID-19 vaccines to be developed include the nucleic acid platform vaccines from Pfizer/BioNTech and Moderna, as well as the adenovirus vector vaccines from AstraZeneca and Johnson & Johnson. All vaccines have been confirmed to be capable of reducing the risk of COVID-19 related hospitalization by 71% or more.
Despite the intensive deployment of these vaccines and many others, just over a third of the world’s people have been fully vaccinated. In addition to the aforementioned challenges, vaccine hesitancy due to doubts about vaccine safety stemming from the unprecedented speed of development, as well as the use of novel platforms, has also affected vaccine rollout.
The finding that an existing, commonly used, and trusted vaccine had the ability to induce humoral immunity against SARS-CoV-2 would therefore be extremely gratifying.
The rationale for looking for anti-SARS-CoV-2 antibodies among those elicited by other vaccines comes from early data collected during the vaccine development effort. This data was based on the hypothesis that vaccines to viruses that have structural or mechanistic similarities to this pathogen might induce antibodies to it.
This applies specifically to the poliovirus vaccine, with its non-structural protein nsp12, also called ribonucleic acid (RNA)-dependent RNA polymerase (RdRp). This enzyme is essential for all RNA viruses, as it is needed for the synthesis of the viral RNA and viral replication. The poliovirus RdRp is closely related to the SARS-CoV-2 nucleoprotein, which is necessary for viral assembly and replication.
As such, researchers have tried to develop specific antiviral drugs that target the RdRp, including remdesivir. In the current paper, this possibility is further explored.
Antibodies to the SARS-CoV-2 RdRp bind to the poliovirus RdRp, called 3Dpol, at one or more sites. Since the 3Dpol protein is incorporated in the polio vaccine, it is likely that this vaccine could effectively support the immune response against SARS-CoV-2. A recent serological study showed that indeed, antibodies to SARS-CoV-2 may be induced by the poliovirus vaccine.
In other words, the inactivated polio vaccine (IPV) elicits heterologous immunity such that memory T-cells can target epitopes shared between the poliovirus and SARS-CoV-2. Therefore, there is a possibility that the IPV can induce an effective immune response against SARS-CoV-2 and prevent viral replication, thus indicating that the SARS-CoV-2 RdRp is another target for antibody and vaccine development.
Study findings
The current study included 300 participants, with a mean age of 51 years, almost equally split between males and females. None of these individuals had a history of COVID-19 or of taking any COVID-19 vaccine. None had taken the poliovirus vaccine within the last 12 years.
All received a dose of the IPV intramuscularly. On day 28, their blood was tested for poliovirus antibodies, including anti-3Dpol antibodies, and the participants’ samples were compared to baseline samples collected before the immunization. No serious side effects were reported from the immunization within the first week after the shot.
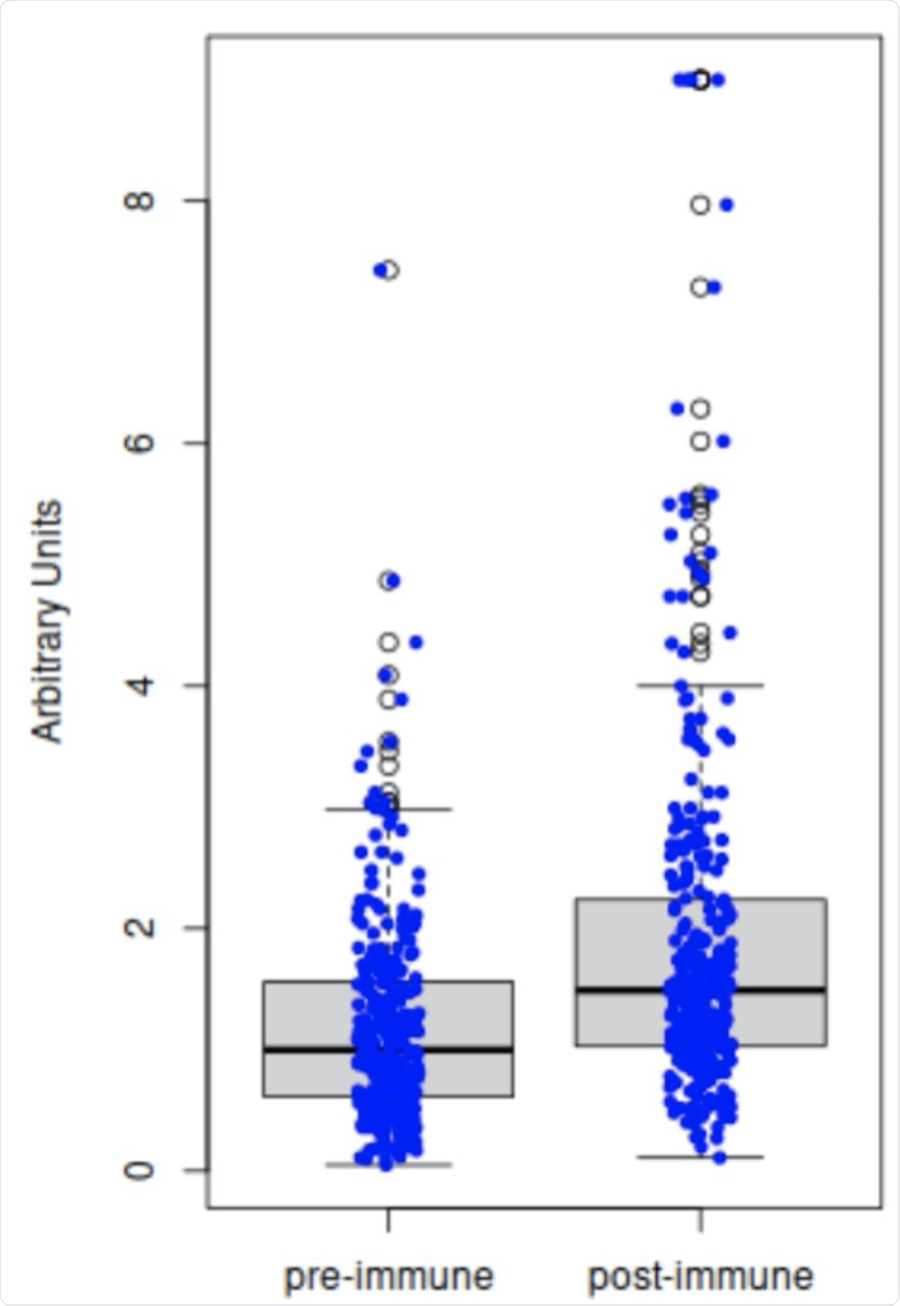 Comparison of antibody titers pre-inoculation (Day 1) versus post-inoculation (Day 28±3). The results from ELISA of the 298 paired serum samples demonstrate that inoculation with inactivated poliovirus vaccine (IPV) significantly increases the titers of anti-3Dpol (RNA-dependent RNA polymerase, RdRp) antibodies in adults (two-tailed t-test, p<0.001).
Comparison of antibody titers pre-inoculation (Day 1) versus post-inoculation (Day 28±3). The results from ELISA of the 298 paired serum samples demonstrate that inoculation with inactivated poliovirus vaccine (IPV) significantly increases the titers of anti-3Dpol (RNA-dependent RNA polymerase, RdRp) antibodies in adults (two-tailed t-test, p<0.001).
Five participants, or less than 2%, tested positive for COVID-19 within the 28-day period; however, only mild symptoms such as fever, tiredness, and a sore throat were reported, all of which resolved within 3 days. As of date, no hospitalizations or deaths have been reported.
Anti-3Dpol antibodies increased from the baseline over the next 28 days in 85% of participants, while 12% showed a decline. About 2% were unchanged because they had high antibody levels at baseline.
The potential inhibition of SARS-CoV-2 RNA synthesis was also assessed in terms of the blocking activity of the serum against 3Dpol.
Of the approximately 300 post-vaccination samples, 54 were randomly tested for the SARS-CoV-2 RdRp polymerase activity. To this end, SARS-CoV-2 RdRp polymerase activity was downregulated in 94% of the participants, thus demonstrating that post-IPV, viral RdRp synthesis was inhibited. In the other three cases, RdRp values were increased.
Despite the individual variation, IPV shows its ability to reduce the risk of progressive disease in COVID-19-positive cases. Only three of the 54 individuals were likely to have viral replication that could lead to symptomatic illness.
“These results show polio-immune sera are very likely to inhibit SARS-CoV-2 RNA synthesis to some extent, thereby affecting the viral replication process.”
Implications
The findings of the current study show that IPV enhances anti-3Dpol activity in adults, especially when their baseline titers are low. Sera containing significant anti-poliovirus antibodies were able to inhibit the synthesis of SARS-CoV-2 RNA.
The study also demonstrates that IPV can elicit antibodies to the poliovirus at high titers in adults, with all participants registering an increase in antibody titers at baseline, except for those who already had high pre-existing titers.
Immune sera with polio antibodies were able to block SARS-CoV-2 RNA synthesis, thus preventing viral replication. Over 95% of the sera showed such inhibitory activity. This outcome is likely to be extremely valid in the current pandemic.
The results of the current study corroborate the findings of an earlier serologic study which showed that adults who had received the IPV had increasing titers of anti-polio RdRp (3Dpol) antibodies that were cross-reactive with the SARS-CoV-2 RdRp protein. If confirmed by a randomized controlled trial, this could mean a valid replacement of expensive mRNA and adenovirus vector vaccines with the much less costly and shelf-stable IPV for the prevention of COVID-19.
Most of the world’s population has already been immunized against poliovirus as a part of childhood immunization programs. However, the very low antibody levels in adults are not likely to be protective against COVID-19, making the findings of this study applies only to those who have received an IPV shot within the last 28 days.
Importantly, the study cohort represented a broad spectrum with underlying medical disorders, the only criteria being the absence of active infection with any pathogen and the absence of pregnancy. This was possible due to the fact that IPV is a fully approved vaccine that is known to be extremely safe.
“This clinical trial was the first study of its kind to evaluate IPV in the context of the COVID-19 pandemic.”
Further studies will be needed to assess the degree of protection in different subgroups and against different SARS-CoV-2 variants. Future studies may also include a larger population at multiple centers for greater generalization. This may help understand how the immune response to IPV occurs in a variety of settings, across multiple age groups and ethnic origins.
A comparison of the sera from individuals who have received the COVID-19 vaccines and/or the IPV vaccine would also be useful, along with a placebo arm. The duration of the raised antibody titers should also be assessed after one, two, or three doses of IPV.
“This readily-available vaccine could be an invaluable means for protection in resource-deficient countries that may not have access to other COVID-19 vaccines. While larger studies should further validate this claim, the preliminary data from this study reinforce findings from a prior retrospective study. Additionally, this study encourages stronger consideration of using IPV as a potential therapeutic agent that is more readily available in resource-deficient countries, as well as a well-established vaccine that may be viewed as a safer and more widely trusted option for vaccine-hesitant populations.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Communale, B. A. et al. (2021). Inactivated Poliovirus Vaccine Induces Antibodies that Inhibit RNA Synthesis of SARS-CoV-2: An open-label, pre-post vaccine clinical trial. medRxiv. doi:10.1101/2021.10.05.21264598. https://www.medrxiv.org/content/10.1101/2021.10.05.21264598v1
- Peer reviewed and published scientific report.
Comunale, Brittany A., Lilly Engineer, Yong Jiang, John C. Andrews, Qianna Liu, Lyuqing Ji, James T. Yurkovich, Roderick A. Comunale, and Qiyi Xie. 2021. “Poliovirus Vaccination Induces a Humoral Immune Response That Cross Reacts with SARS-CoV-2.” Frontiers in Medicine 8: 710010. https://doi.org/10.3389/fmed.2021.710010. https://www.frontiersin.org/articles/10.3389/fmed.2021.710010/full.