Future work is needed to see if the reduction in vaccine protection translates to reduced protection against hospitalization and death. As a result, having multiple forms of protection, including booster shots and following public health measures, may help with preventing and transmitting infection.
“COVID-19 vaccines remain the most important tool to prevent infection, severe illness, and death, but vaccines should be accompanied by additional measures, including masking, hand washing, physical distancing, and other public health interventions, in the face of increased risk of infection due to the Delta variant,” explained the researchers.
Study details
The researchers looked at the number of SARS-CoV-2 infections amongst U.S. veterans who received care via the Veterans Health Administration. They observed infection rates that took place from February 1, 2021, to August 13, 2021 — when vaccines became available to U.S. veterans.
As of mid-August 2021, approximately 3.3 million veterans are fully vaccinated.
The VA Corporate Data Warehouse was used to collect information on veterans’ vaccination status and the type of vaccine given, if applicable. Veterans who received partial vaccination or vaccinations not approved by the U.S. Food & Drug Administration (FDA) were not included in the final analysis.
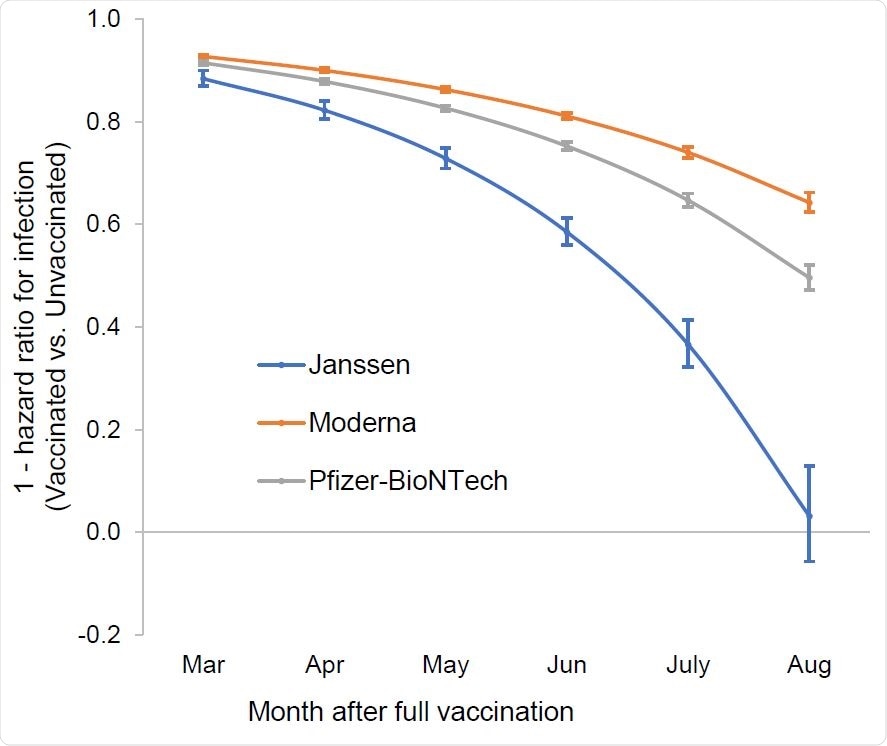
Time dependent vaccine protection against SARS-CoV-2 infection as estimated from Cox proportional hazards models, adjusted for age, race, ethnicity, sex and comorbidity Associations are presented as 1 – hazard ratios and 95% confidence intervals. Associations for each month were estimated from contrasts using product terms for vaccination status by time to most recent RT-PCR.
Factors influencing COVID-19 infection rates
The risk of infection was highest among unvaccinated Veterans — about 16.6% tested positive on a PCR test for COVID-19.
Other common factors shared among veterans positive for COVID-19 included being female (10.2%) and being older than 50 (13.6%).
People who identified as Hispanic, American Indian/Alaska Native, or Native Hawaiian/Pacific Islander were more likely to have COVID-19 infection.
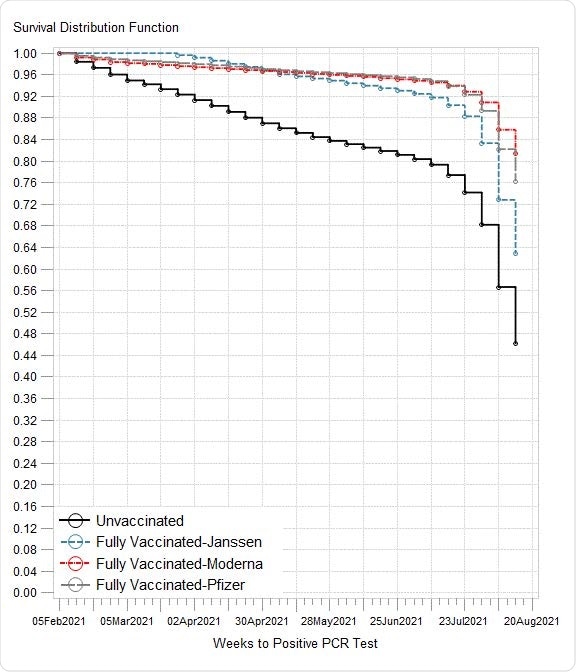
Kaplan-Meier curves illustrating risk of SARS-CoV-2 infection by vaccination status and age: A) all ages; B) age <50 years, C) age 50-64 years; D) age ≥65 years The survival function estimates time to infection detected by most recent RT-PRC.
Waning immunity and the rise of Delta
All FDA-approved COVID-19 vaccines reduced the risk of infection in early 2021.
But, all vaccines showed reduced protection over time, with the most significant decline in protection correlating with an increase in infections observed in July and August 2021. The researchers note the increased infections overlapped with the spread of the Delta variant in the summer.
“Increasing risk of infection was not explained by age or comorbidity, implicating increased infectivity of the Delta variant vs. waning immunity as the primary determinant of infection,” explained the researchers.
Johnson & Johnson showed the largest decline
The largest decline in vaccine protection came from the Johnson & Johnson vaccine.
Initially, the Johnson & Johnson vaccine was 88% effective in preventing infection. But by August, vaccine effectiveness dropped to 3%.
For the Pfizer-BioNTech vaccine, vaccine protection started at 91% and was reduced to 50% protection by August.
The Moderna vaccine showed the smallest reduction in vaccine effectiveness. A 92% vaccine effectiveness decreased to 64% by August.
“The proportional reduction in vaccine effectiveness against infection across all age groups underscores the critical importance of a layered approach to protection, while enhancing continued efforts to increase vaccination among the 90 million Americans who remain unvaccinated,” conclude the researchers.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Cohn B.A., et al. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans, February 1, 2021 to August 13, 2021. medRxiv, 2021. Doi: https://doi.org/10.1101/2021.10.13.21264966, https://www.medrxiv.org/content/10.1101/2021.10.13.21264966v1
- Peer reviewed and published scientific report.
Cohn, Barbara A., Piera M. Cirillo, Caitlin C. Murphy, Nickilou Y. Krigbaum, and Arthur W. Wallace. 2021. “SARS-CoV-2 Vaccine Protection and Deaths among US Veterans during 2021.” Science, November. https://doi.org/10.1126/science.abm0620. https://www.science.org/doi/10.1126/science.abm0620.