Widespread vaccination campaigns are currently in progress around the world in an effort to control the coronavirus disease 2019 (COVID-19) pandemic. While vaccination poses an appreciable prophylactic measure to prevent the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the scientific community is still struggling to develop a robust treatment to cure already infected COVID-19 patients.
Although vaccines were rapidly developed and marketed with unprecedented swiftness, drug development has traditionally been much more time extensive. Considering the urgency, drug repurposing has emerged as a promising strategy that is already being followed or tested in several preclinical and clinical trials.
In a recent review published in the Journal of Cellular Biochemistry, the author Dr. Theo Rein discusses autophagy as a potential contributing mechanism of selected drugs that are currently being evaluated under the World Health Organization (WHO) Solidarity program in the treatment of COVID‐19.
Autophagy and its manipulation by coronaviruses
Autophagy is an evolutionarily conserved intracellular self-degradative process that plays a crucial housekeeping role in removing misfolded proteins, clearing damaged organelles, as well as eliminating intracellular pathogens, including viruses.
During autophagy, cellular elements destined for degradation are engulfed by a double‐membrane structure called an autophagosome. These autophagosomes eventually fuse with lysosomes producing autolysosomes, where degradation takes place. The rate of autophagic degradation through this degradative cascade is known as “autophagic flux.”
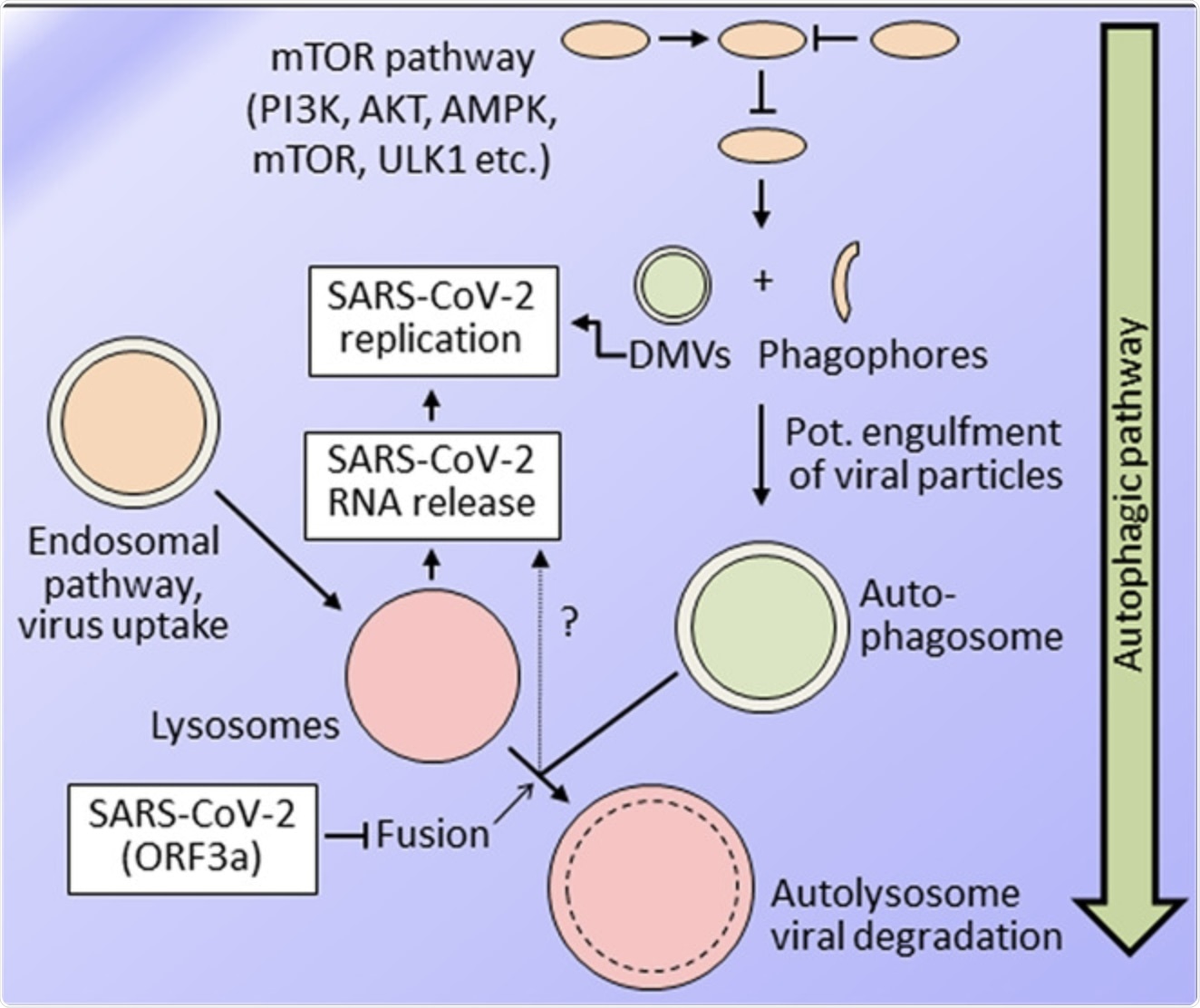 Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) replication and endosomal/autophagic pathways, simplified scheme. Current knowledge supports both beneficial and detrimental effects of the autophagic pathway for SARS-CoV-2 replication. A major entry route for the virus is endocytic uptake, which requires lysosomal acidification for viral RNA release. The autophagic pathway is a multifactorial and multistep pathway with a vast range of possibilities for pharmacological targeting. In the more initial phases, phagophores, and double-membrane vesicles (DMVs) are formed, most likely from the endoplasmic reticulum, possibly also promoted by some coronavirus proteins. SARS-CoV-2 replication takes place at the endoplasmic reticulum as well, at very similar, if not identical, membrane structures. SARS-CoV-2 inhibits the last step of autophagy leading to viral degradation, that is, the fusion of autophagosomes with lysosomes to form autolysosomes, thus inhibiting autophagic flux. Accordingly, compounds impacting autophagy are expected to be efficient in fighting SARS-CoV-2 only if they enhance autophagic flux
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) replication and endosomal/autophagic pathways, simplified scheme. Current knowledge supports both beneficial and detrimental effects of the autophagic pathway for SARS-CoV-2 replication. A major entry route for the virus is endocytic uptake, which requires lysosomal acidification for viral RNA release. The autophagic pathway is a multifactorial and multistep pathway with a vast range of possibilities for pharmacological targeting. In the more initial phases, phagophores, and double-membrane vesicles (DMVs) are formed, most likely from the endoplasmic reticulum, possibly also promoted by some coronavirus proteins. SARS-CoV-2 replication takes place at the endoplasmic reticulum as well, at very similar, if not identical, membrane structures. SARS-CoV-2 inhibits the last step of autophagy leading to viral degradation, that is, the fusion of autophagosomes with lysosomes to form autolysosomes, thus inhibiting autophagic flux. Accordingly, compounds impacting autophagy are expected to be efficient in fighting SARS-CoV-2 only if they enhance autophagic flux
The entire process of autophagy is tightly controlled and executed by a vast array of proteins. Given its involvement in various pathological conditions including viral infection, scientists across the world are trying to develop drug compounds that target proteins in the autophagic cascade.
Double‐membrane structures derived from the endoplasmic reticulum, which are typically required for the initial steps of autophagy, are contrived to serve as replication sites by coronaviruses. In addition, non-structural proteins of coronaviruses induce the formation of these double‐membrane structures.
Besides maneuvering early steps, coronaviruses can also interfere with the late steps of autophagy to evade degradation. Regarding SARS-CoV-2, the ORF3a protein has recently been reported to inhibit the fusion of autophagosomes with lysosomes. As a result, ORF3a increases autophagosomes but decreases autophagic flux, thus providing an escape from autophagy. Therefore, the author emphasizes autophagy drugs to target promoting autophagy flux rather than other aspects of the cascade.
Taken together, coronaviruses benefit from the early steps of the autophagic pathway but are vulnerable to increased autophagic flux. This finding is supported by previous reports showing that induction of autophagy has the potential to fight coronavirus infection.
“As autophagy is a conserved mechanism operative in most cells, pharmacological induction of autophagy has the potential to fight SARS‐CoV‐2 in all organs that are reached by the compound.”
“Solidarity” initiative by WHO to test repurposed drugs for COVID-19
The WHO initially launched the research program “Solidarity” in 2020 to test four compounds as options for antiviral treatment, namely remdesivir, interferon β1a (IFN- β1a), hydroxychloroquine, and a combination of lopinavir and ritonavir. Unfortunately, an interim report of the study including 11,330 COVID-19 patients revealed little or no effect.
A more recent initiative in the Solidarity program evaluates three established immunomodulatory drugs including infliximab, imatinib, and artesunate. These drugs are selected on the rationale of restricting the damage caused as a result of an exaggerated immune response or cytokine storm, rather than trying to fight the SARS-CoV-2 directly.
The author of the current study points to the potential involvement of autophagy in the action of these drugs, which may play a more prominent role than generally acknowledged.
Artesunate
Artesunate is a derivate of artemisinin that has established antimalarial and potent anticancer effects. This drug was added to the WHO’s Solidarity program because of its effects on the immune system. Several studies have reported the mechanism of autophagy induction by artesunate, with some reporting enhanced autophagy flux as well.
Infliximab
Infliximab is a chimeric antibody targeting tumor necrosis factor α (TNF-α) used in clinical practice to treat autoimmune diseases like Crohn's disease. Recent reports on drugs including infliximab that are either approved or in a clinical trial to treat Crohn's disease suggest autophagy induction as a relevant mechanism contributing to its effect. However, more studies are needed to specifically investigate the effect of infliximab on autophagy.
Imatinib
Imatinib is an ABL tyrosine kinase inhibitor used to treat chronic myeloid leukemia. Several studies report an autophagy-inducing effect of imatinib, including the assessment of autophagic flux. Nevertheless, more studies are needed that apply a broader range of autophagic flux assessments to solidify the conclusion that imatinib induces autophagy.
Other autophagy-inducing drugs with anti-SARS-CoV-2 potential
Antidepressants
There is growing evidence that COVID-19 patients benefit from antidepressant treatment. Studies on COVID-19 patients receiving antidepressants have shown a reduced risk of intubation and deaths, reduced hospitalizations, and a lower likelihood of clinical deterioration. In fact, one recent preclinical study found the antidepressant fluoxetine as an inhibitor of SARS‐CoV‐2 in human lung tissue.
Antidepressants are known to exert their beneficial effects by reducing the risk of the often fatal cytokine storm. However, antidepressants induce autophagy as well. Thus, their effect on autophagy might not only be important for treating depression but also to fight SARS-CoV-2.
Ivermectin
Ivermectin is an anthelmintic macrolide of the avermectin group and has been investigated as a potential anti‐SARS-CoV-2 agent with promising initial results. Several mechanisms are discussed for its apparent antiviral activity. However, the author argues for adding autophagy to this panel, as a growing number of studies report an autophagy‐inducing effect of ivermectin, including one study that carefully determined autophagic flux.
Conclusion
The author highlights the possibility that the three drugs recently added to the WHO Solidarity program may not just prevent a life-threatening cytokine storm during SARS-CoV-2 infection but may also potentially limit SARS-CoV-2 replication through activating autophagy.
“The interaction of SARS‐CoV‐2 with the autophagic pathway is complex, with evidence for both the virus taking advantage of the autophagic pathway and trying to tame the full activity of this pathway to prevent its degradation. It is obvious from this scenario that it will be essential to learn how exactly the SARS‐CoV‐2 life cycle is intertwined with the autophagic pathway.”