The coronavirus disease (COVID-19) pandemic, caused by the SARS-CoV-2, still rages in many countries around the world, putting significant strains on health systems and economies around the world. One of the main steps towards the end of the pandemic is the use of vaccines.
In a nutshell, COVID-19 vaccines elicit the production of antibodies that are directed against the viral spike glycoprotein, which in turn neutralize the virus. A similar antibody response is seen after natural SARS-CoV-2 infection and these antibodies aid in the protection against severe forms of the disease and death.
Nevertheless, the emergence of SARS-CoV-2 variants that harbor mutations in the spike glycoprotein may resist neutralization by antibodies, compromising vaccine efficacy. Moreover, emerging viral variants with improved transmission properties (most likely due to modified virus-host cell interactions) might swiftly spread globally.
This is why a research group from Germany (led by Dr. Prerna Arora from the Georg-August-University Göttingen) decided to investigate antibody-mediated neutralization and cell entry specific for variant A.30 (also known as A.VOI.V2) detected in spring 2021 in several patients in Angola and Sweden, with likely origins from Tanzania.
In vitro comparisons of SARS-CoV-2 variants
In order to analyze viral cell entry and its inhibition by antibodies, the researchers have employed rhabdoviral pseudotypes with SARS-CoV-2 spike glycoprotein. As targets, they have used kidney-derived 293T and Vero cells, lung-derived A549 and Calu-3 cells, liver-derived Huh-7 cells, and colon-derived Caco-2 cells.
Furthermore, in order to compare A.30 with other SARS-CoV-2 variants, the scientists have analyzed Beta (B.1.351) and Eta (B.1.525) variants. The reason was that these two variants were initially discovered in Africa (akin to A.30), and B.1.351 is considered a salient variant of concern – with the highest level of neutralization resistance among all the variants known to date.
It also has to be emphasized that, in comparison to the spike glycoprotein of the SARS-CoV-2 B.1 variant that was known to circulate during the early phase of the COVID-19 pandemic, the spike glycoprotein of the A.30 variant carries ten amino acid substitutions and five deletions.
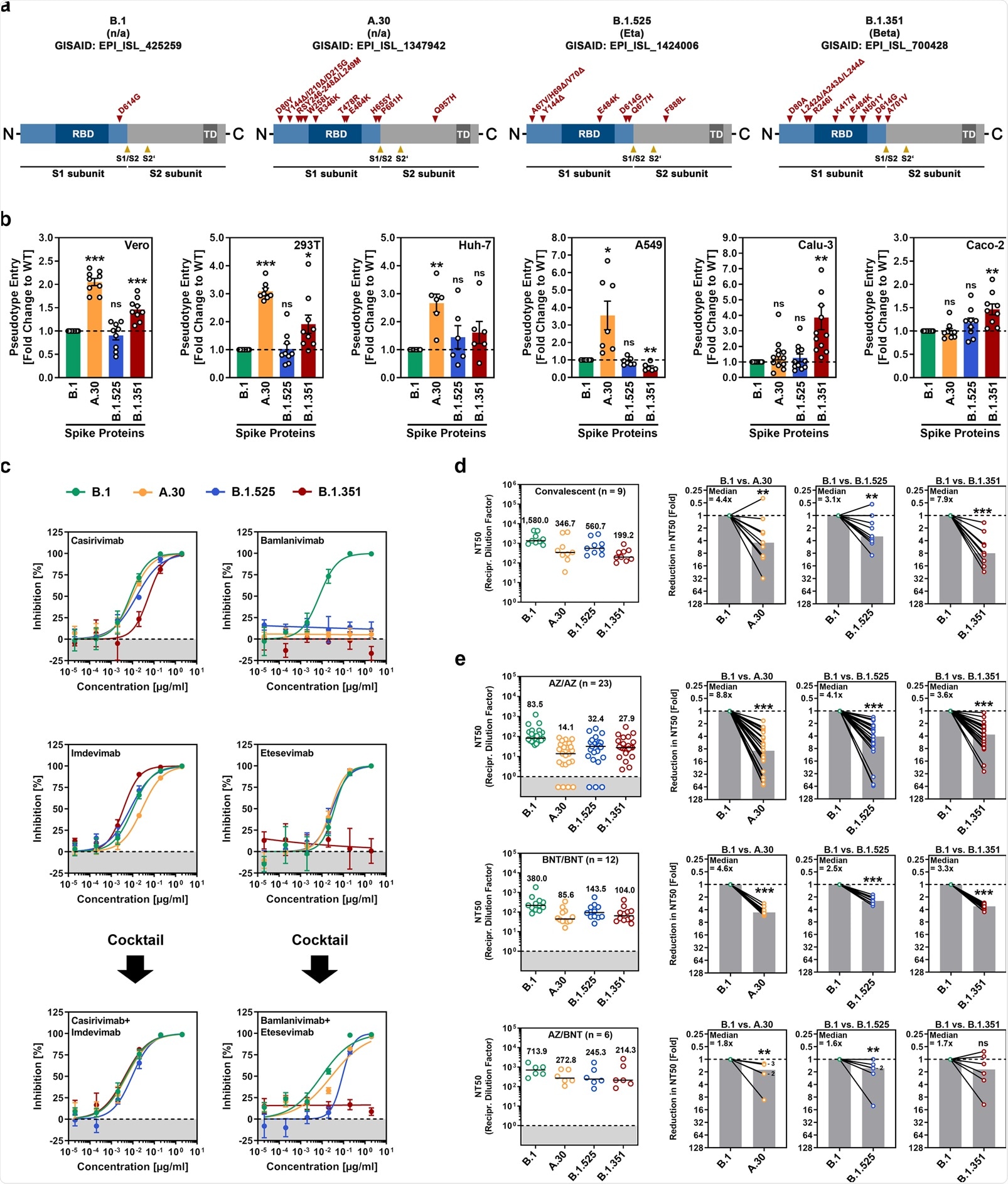
SARS-CoV-2 A.30 enters certain cell lines with increased efficiency and evades antibody-mediated neutralization. a Schematic overview and domain organization of the SARS-CoV-2 S proteins studied. Abbreviations: RBD, receptor-binding domain; TD, transmembrane domain. b Pseudotyped particles bearing the indicated S proteins were inoculated onto different cell lines, and transduction efficiency was quantified by measuring virus-encoded luciferase activity in cell lysates at 16–18 h postinoculation. Presented are the average (mean) data from six to 12 biological replicates (each conducted with technical quadruplicates) for which transduction was normalized against SARS-CoV-2 S B.1 (= 1). Error bars indicate the standard error of the mean (SEM). The statistical significance of differences between B.1 and A.30, B.1.525, or B.1.351 was analyzed by two-tailed Student’s t-test with Welch correction (p > 0.05, not significant [ns]; p ≤ 0.05, *; p ≤ 0.01, **; p ≤ 0.001, ***). See also Supplemental information, Fig. S1b. c Neutralization of SARS-CoV-2 B.1, A.30, B.1.525, and B.1.351 by monoclonal antibodies used for COVID-19 therapy or an unrelated control antibody (Supplemental information, Fig. S1d). Pseudotyped particles were incubated for 30 min at 37 °C in the presence of increasing concentrations (0.00002, 0.0002, 0.002, 0.02, 0.2, 2 µg/ml) of the indicated monoclonal antibodies or an unrelated control antibody before being inoculated onto Vero cells. Infection efficiency was quantified by measuring virus-encoded luciferase activity in cell lysates at 16–18 h postinoculation. Presented are average (mean) data from a single biological replicate (conducted with technical quadruplicates) for which infection was normalized against samples that did not contain antibody (= 0% inhibition). The data were confirmed in a separate independent experiment. Error bars indicate the standard deviation. d Neutralization of SARS-CoV-2 B.1, A.30, B.1.525, and B.1.351 by antibodies in convalescent plasma. Pseudotyped particles bearing the indicated S proteins were incubated for 30 min in the presence of different dilutions of convalescent plasma (n = 9). Infection efficiency was determined as described for Fig. 1b and used to calculate the plasma dilution factor leading to a 50% reduction in S protein-driven cell entry (neutralizing titer 50, NT50). Data from a total of nine convalescent plasma samples are presented (black lines and numerical values indicate the median NT50). In addition, for each plasma, the fold reduction in NT50 between SARS-CoV-2 B.1 (set as 1) and the indicated variants was calculated (gray bars indicate the median). The statistical significance of differences between the indicated groups was analyzed by a two-tailed Mann–Whitney test with a 95% confidence level (p > 0.05, ns; p ≤ 0.05, *; p ≤ 0.01, **; p ≤ 0.001, ***). e The experiment was performed as described in Panel d, but serum from ChAdOx1 nCoV-19/ChAdOx1 nCoV-19 (AZ/AZ; n = 23), BNT162b2/BNT162b2 (BNT/BNT; n = 12) or ChAdOx1 nCoV-19/BNT162b2 (AZ/BNT; n = 6)-vaccinated individuals was investigated. Numbers in the bar graphs “B.1 vs. A.30” and “B.1 vs. B.1.525” indicate the number of overlapping data points (dots)
Robust cell entry and neutralization resistance
This study has shown that A.30 has a cell line preference that is not seen with other viral variants. Furthermore, this specific variant can efficiently evade neutralization by antibodies that have been elicited by Astrazeneca-Oxford (AZD1222) or Pfizer-BioNTech (BNT162b2) vaccines.
As SARS-CoV-2 entry into cell lines highly depends on the activation of spike glycoprotein by the cellular proteases cathepsin L or TMPRSS2 (with the latter supporting viral spread in the lungs), it is of particular interest that enhanced A.30 entry was seen for cell lines with cathepsin L (i.e., 293 T, Vero, A549 and Huh-7 cells) – but not for TMPRSS2-dependent entry (i.e., Calu-3 and Caco-2 cells).
In addition, such improved cell line entry was combined with notable resistance to antibodies after AstraZeneca-Oxford or Pfizer-BioNTech vaccination. Neutralization resistance of A.30 exceeded that of the Beta (B.1.351) variant, which was already very resistant to neutralization in cell culture and less well inhibited by the AstraZeneca-Oxford vaccine compared to the Alpha variant.
Public health answer to the variant conundrum
Overall, these results imply that the SARS-CoV-2 variant A.30 can successfully evade control by vaccine-induced antibodies and might have an increased capacity to enter cells in a cathepsin L-dependent manner, which might open the door for viral dissemination outside the lungs.
“As a consequence, the potential spread of the A.30 variant warrants close monitoring and rapid installment of countermeasures”, warn the authors of this study.
However, heterologous vaccination with both above-mentioned vaccines was previously shown to boost neutralizing antibody responses against variants of concern compared to corresponding homologous vaccinations; hence, this might offer robust protection against the A.30 variant as well. In any case, further studies are needed to address this critical public health issue.