Inhaled corticosteroids (ICS) are used as first-line treatment modalities in patients with asthma and chronic obstructive pulmonary disease (COPD) to reduce the risk of exacerbations often triggered by extrinsic factors, such as respiratory viruses. It was recently reported that patients with asthma and COPD are less likely to be hospitalized with severe coronavirus disease 2019 (COVID-19). Evidence suggests that patients with severe infection who used ICS two weeks before hospitalization exhibited better clinical outcomes.
The Steroids in COVID (STOIC) trial had enrolled 144 patients, aged 18-79 years of age, with early COVID-19 symptoms. Patients were randomized into a usual care (UC) arm, which consisted of those who were given antipyretics, whereas the second group of patients included those who were provided inhaled budesonide (BUD) at a dose of 800 micrograms (μg) twice a day, along with usual care.
All participants were assessed on day 0 (visit 1), day 7 (visit 2), and day 14 (visit 3). In the STOIC trial, inhaled budesonide, which is a corticosteroid, had been evaluated as a treatment for early COVID-19 infection. The benefits of this treatment approach included the improvement of self-reported symptom recovery, fewer adverse events, and faster symptom resolution.
About the study
The objective of a new study published on the medRxiv* preprint server was to gain an understanding of the mechanism of action of budesonide in improving the prognosis of patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
The present study involved the assessment of inflammatory mediators in the nasal mucosa of patients recruited to the STOIC trial and a cohort of SARS-CoV-2 negative individuals. This study was funded by the Oxford NIHR Biomedical Research Centre and AstraZeneca (Gothenburg Sweden).
The findings of this study depicted the nasal mucosal inflammatory response in patients with early COVID-19, as well as the evolution of inflammation in the natural course of the infection. The results demonstrated how budesonide can ameliorate the exaggerated inflammatory response seen in early COVID-19 and aid in improving clinical outcomes.
Upon comparing the inflammatory profile of nasal mucosal fluid among 20 healthy controls and in patients with early COVID-19, 14 mediators were found to be elevated in COVID-19 patients. In addition, altered T-cell response with significantly reduced levels of thymic stromal lymphopoietin (TSLP) and CCL17, along with reduced CCL2 levels were noted, implying impaired monocyte recruitment. There were also lower levels of vascular endothelial growth factor (VEGF), as previously described in severe hospitalized COVID-19 cases.
Upon examining nasal mucosal inflammation in the BUD arm, during the 14-day course of COVID-19, CXCL9, CXCL10, CXCL11, interleukin (IL)-12, IL-10, IL-2, interferon (IFN)α2a, CCL2, CCL3, CCL4, IL-6, IL-4, CCL13 and tumor necrosis factor (TNF)-α were significantly reduced. Of the 14 mediators elevated in early COVID-19, 11 mediators decreased over time, of which included CCL3, CCL4, TNF-α, IL-6, CCL13, IL-4, IFN-α2a, CXCL10, CXCL11, IL-2, and IL-12. However, CCL11, IFN-β, and IFN-γ showed no significant variation between visits 1 and 3 and remained elevated after 14 days.
Meanwhile, VEGF, TSLP, and CCL17 levels did not change and remained lower than that of healthy controls; however, CCL2 and IL-10 levels were significantly reduced. Similarly, IL-1β, CXCL8, IL-33, CCL24, CCL26, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) did not show any changes over time, nor did the mediators PDGFA, CCL5, and TGFβ1. Notably, the levels of the chemokine CXCL9, reduced with time.
Additionally, the concentrations of 30 mediators were examined in paired visit 1 and visit 3 samples in the BUD arm. The results showed that CCL5 was significantly reduced, whereas GM-CSF was not reduced. Concentrations of IL-2 and IL-4 were sustained in the BUD study arm, unlike in the UC study arm.
Upon correlating the viral load with type I IFN expression and inflammatory markers of the nasal mucosa, differences between local and systemic inflammation were marked, which were speculated to be related to the number of viral particles. While assessing whether the inflammation associated with SARS-CoV-2 infection declined over time, it was found that although certain mediators reduce during the disease, multiple mediators remain elevated within the nasal mucosa, even much after clinical recovery.
In COVID-19, the peak inflammatory response generally occurred early in the disease course. Inhaled budesonide suppressed kinetic inflammation, specifically, the secondary increase in inflammation after the 15th day.
However, heightened IFN-γ levels were recorded in the BUD arm at day 15 after symptom onset. When the kinetics of nasal mucosal inflammation were investigated over the first seven days of symptom onset, a high eosinophilic phenotype correlated with severe disease.
To understand any aberrant immune response during the early part of SARS-CoV-2 infection, a comparison of mediators in individuals with COVID-19 and in those who clinically deteriorated was performed. To this end, in the early stages of the disease, a blunted response to infection occurs in patients who deteriorate.
In the BUD arm, serum inflammatory mediators remained elevated up to 35 days after the initial infection. Systemic inflammation was suppressed to a greater degree by inhaled budesonide, thereby preventing long COVID from developing.
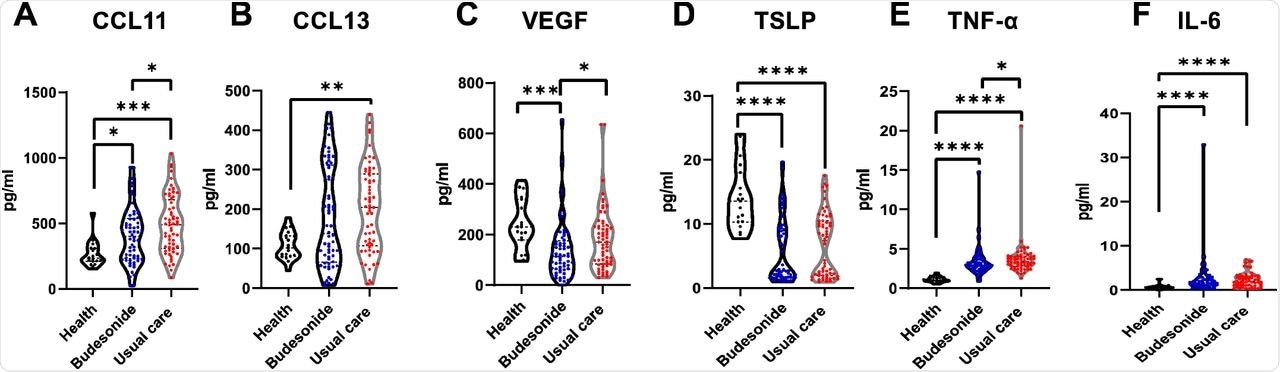 Persistence of systemic inflammation following 28-35 days of COVID-19 infection in the community, is dampened by inhaled budesonide. A-F) Violin plots comparing some mediator levels in the serum of healthy individuals (n=19), those in the usual care arm (n=60) and budesonide arm (n=62) of the study at 28-35 days following COVID-19 infection (see supplement S7 for further results). Data were analysed by Kruskal-Wallis with post-hoc Dunn’s test. * p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001.
Persistence of systemic inflammation following 28-35 days of COVID-19 infection in the community, is dampened by inhaled budesonide. A-F) Violin plots comparing some mediator levels in the serum of healthy individuals (n=19), those in the usual care arm (n=60) and budesonide arm (n=62) of the study at 28-35 days following COVID-19 infection (see supplement S7 for further results). Data were analysed by Kruskal-Wallis with post-hoc Dunn’s test. * p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001.
Network analysis demonstrated that there was a 72% overlap between the inflammatory response at an early and at the later timepoint in COVID-19, thus indicating persistent inflammation of similar pathways. In the BUD arm, the nasal mucosal response varied, with an increased anti-inflammatory response (IL-10) and alarmins (IL-33 and TSLP) and a reduced T2 inflammatory response, thus suggesting suppression of the T2 hyper-inflammatory response with COVID-19.
These results indicated that the ICS treatment of budesonide modulates inflammatory pathways in the upper respiratory tract and in circulation in patients with COVID-19 by decreasing the transcription of inflammatory cytokines. Therefore, the findings support the therapeutic mechanism of corticosteroids in the management of SARS-CoV-2 infection.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.