In this interview, News-Medical talks to Dr. Dana Fletcher, Martin Berner, and Robin Pettini from Siemens Healthineers about the connection between high-sensitivity troponin and heart attacks.
What is high-sensitivity troponin, and how is it connected to the detection of heart attacks?
High-sensitivity troponin is defined as the ability to detect cardiac troponin concentrations with a coefficient of variation <10 % at or the below the 99th percentile URL and measurable in >50% of normal healthy individuals.
In other words, high-sensitivity troponin assays can precisely detect very low levels of troponin. We know troponin is released into the bloodstream when cells from the heart die, and specifically the Fourth Universal Definition of Myocardial Infarction defines a heart attack as, when blood levels of cardiac troponin are increased above the 99th percentile upper reference limit in conjunction with other criteria.
Image Credit: Shutterstock/Kateryna Kon
What is the difference between contemporary troponin and high-sensitivity troponin?
The major difference between contemporary and high-sensitivity troponin is the ability of high-sensitivity troponin to measure lower levels of troponin, below the percentile that would be considered the concentration for the diagnosis of a heart attack. In addition, high-sensitivity troponin has significantly greater early sensitivity and NPV for the diagnosis of heart attacks.
Due to the higher sensitivity and diagnostic accuracy, the European Guidelines that address this patient population recognize that the time interval to the second cardiac troponin assessment can be shortened with the use of high-sensitivity troponin, which reduces the time to diagnosis and length of stay in the emergency department. In addition, high-sensitivity assays are most useful in borderline situations with marginal elevations of troponin. Finally high-sensivity cardiac troponin I facilitates the use of gender specific reference ranges.
Can you explain what point of care testing (POCT) is and how it benefits the patient?
Point of care testing is testing that is available right where the patient is located and designed to be preformed by the clinical staff. For example, this could be testing done in the emergency department by nurses, or by physicians in the doctor’s office.
Can you describe some of the benefits that POCT seems to have over central lab testing?
The benefit of POCT is decreasing the time for critical lab results to be provided to clinicians. Most often, the laboratory does a great job at processing samples once they arrive in their domain. Unfortunately, it can be a complicated and timely process in the hospital setting for samples from the clinical area to find their way to the lab, known as the pre-analytical process.
POCT diminishes the pre-analytical portion and decreases the turnaround time for lab results. This provides the clinicians important information to assess the patient’s medical condition faster and expedite next steps in the patient’s care pathway, either additional testing and admission, or the potential for earlier discharge. When it comes to the diagnosis of a heart attack, clinicians want to know as quickly as possible to determine treatments, decide appropriate levels of care, and the potential for interventions.
In addition, appreciating that many emergency departments are overcrowded, decreasing length of stay is an important issue to emergency medicine clinicians and hospital administrators alike.
And in the physician office, POCT allow the clinicians to have important lab results while they are assessing and educating patients during that patient appointment, with no need to try to track down important lab results and the potential to reduce call backs.
Why is POCT so important when it comes to myocardial infarction (MI)?
Chest pain is a major complaint of patients presenting to emergency departments. While most patients who have chest pain are not having a heart attack, it is important to quickly determine who is, and troponin is part of that diagnostic assessment.
By decreasing the time to result for troponin with the use of POCT, critical pieces to the puzzle are expedited. Equally important is to assess quickly who is not having a heart attack and determining the next steps faster, for that patient’s journey as well.
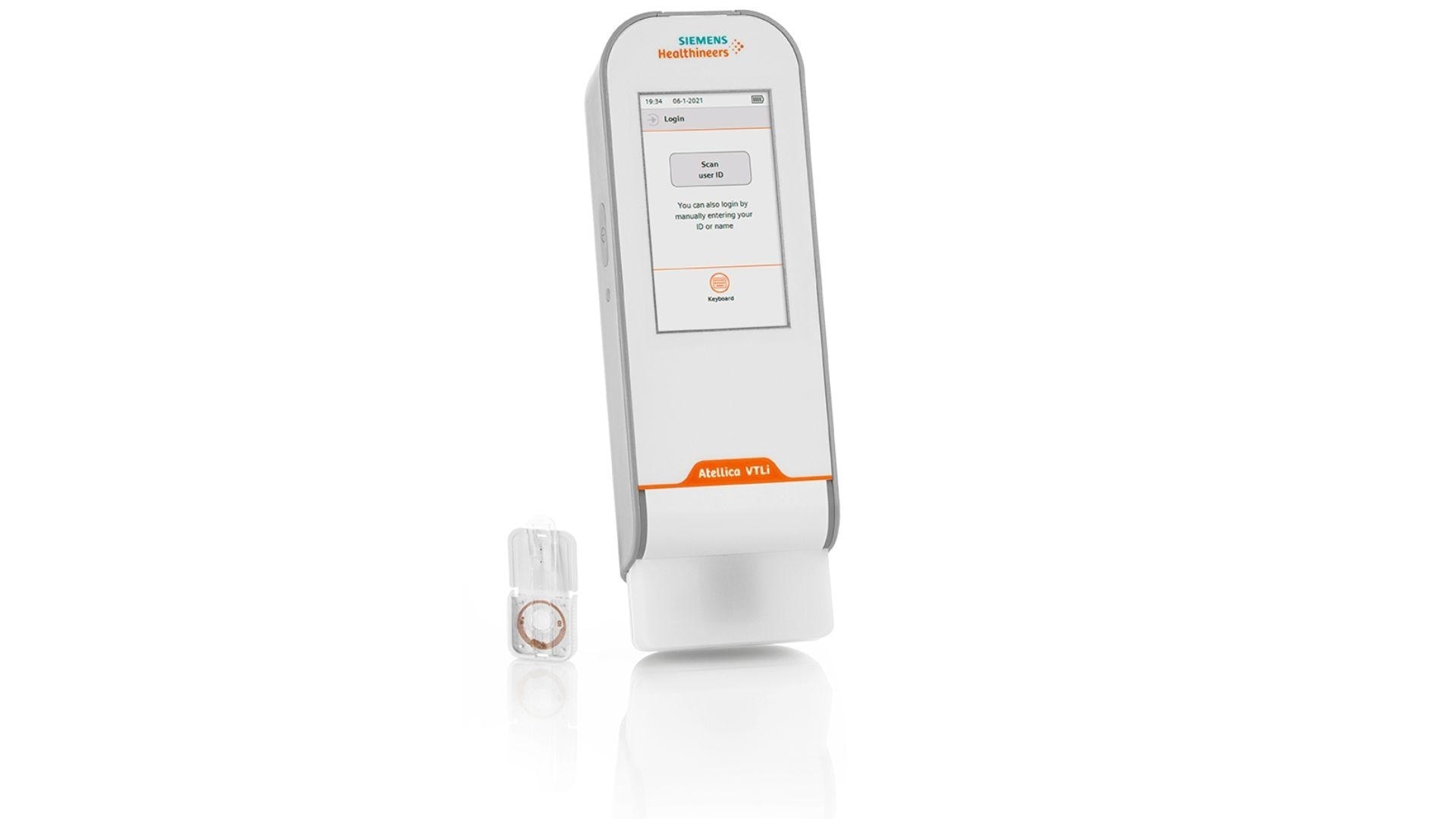
Can you describe some of the features of the Atellica® VTLi Patient-side Immunoassay Analyzer?
On the outside, Atellica VTLi Patient-side Immunoassay Analyzer appears like many other point of care devices on the market today. It’s a lightweight, handheld analyzer with a user interface that’s easy to understand, with easy to follow onscreen instructions.
But it’s what inside the analyzer that really sets the Atellica VTLi system apart from the competition, specifically the measurement and detection systems driven by Magnotech® Technology. Magnotech Technology drives the analytical performance required for the tests that can be run on the analyzer.
What makes this device a "first" in POCT?
There are other systems on the market that offer a high-sensitivity troponin I, but they lack the performance evidence on the sample type most important to the target customer in the Emergency Department, and that sample is whole blood.
The Atellica VTLi high-sensitivity troponin I assay has published data on whole-blood specimens. We also have evidence showing strong correlation between sample types, including whole-blood, plasma, and capillary samples. No other system currently on the market has that flexibility or evidence.
What benefits can the Atellica VTLi Patient-side Immunoassay Analyzer provide over other platforms?
Our system has evidence-based accurate performance on our flagship test: high-sensitivity troponin I that can be performed on a variety of sample types, including fingerstick, and provide results in 8 minutes. Our system may lead to improved throughput in the Emergency Department as it can be easily integrated into current assessment processes.
How does the Atellica VTLi Patient-side Immunoassay Analyzer deliver high-sensitivity cardiac troponin I test results?
The accuracy and precision demanded of a hs-cTnI assay are driven by Magnotech Technology. This technology is based on the use of magnetic nanoparticles to concentrate, separate, and detect the target molecules. The use of magnetic nanoparticles also speeds up the reaction kinetics and enables results to be displayed in only 8 minutes.
Image Credit:Shutterstock/peterschreiber.media
What would you say to clinicians concerned about integrating this technology into their current setup?
For clinicians looking to integrate both high-sensitivity troponin I and POCT into their process, we would suggest looking to the experts. Many key leaders in the field of emergency medicine, laboratory medicine, and cardiology have published guidance on areas to consider when implementing high-sensitivity troponin I and POCT testing.
We would also suggest clinicians, laboratorians, and clinical chemists should inquire from manufactures regarding the technical and clinical training, and support they will provide when considering the implementation of new high-sensitivity POCT technology.
What does the future hold for POCT, and what role will Siemens Healthineers play in this important developing field?
Looking back to where point of care testing was back in the early 1990’s with fingerstick glucose and pregnancy testing, to where we are today, with the first point of care high-sensitivity Troponin I that can be performed from a capillary specimen in 8 minutes is amazing, and we're excited to see where point of care testing goes next.
While we can’t predict the future, we're confident with the dedicated scientists and researchers at Siemens Healthineers, will continue to push the technology and set new benchmarks.
About Siemens Healthineers
Siemens Healthineers develops innovations that support better patient outcomes with greater efficiencies, giving providers the confidence they need to meet the clinical, operational, and financial challenges of a changing healthcare landscape. As a global leader in medical imaging, laboratory diagnostics, and healthcare information technology, we have a keen understanding of the entire patient care continuum—from prevention and early detection to diagnosis and treatment.
About Dr. Dana R Fletcher

Dana R Fletcher, Ph.D., has been in the medical device industry for over 25 years, with experience in cardiology, vascular surgery, transfusion medicine, laser technology, and infectious disease therapeutic areas, primarily in Class III and in vitro diagnostic sectors. She’s served at the research bench, in intellectual property, and in clinical affairs as both an employee and, since 2009, as an independent consultant.
She provides analytic and compliance expertise for companies at the start-up phase to global leaders and has specialized in medical writing and editing in recent years. Her PhD research, completed in 2019, is focused on evaluating the effects of Medicare payments and policies on the utilization of cardiac rehabilitation. In her spare time, she enjoys the great Colorado outdoors.
About Martin Berner
Martin Berner B.Sc is the Head of the Critical Care product portfolio at Siemens Healthineers Point of Care organization. He has worked in the point of care diagnostics field and with organizations ranging from start-up enterprises to multi-national IVD companies for over two decades.
Along with his extensive experience in the POC IVD segment, Martin’s primary passion is to continue to advance patient care by empowering clinicians and the laboratory with point-of-care technology solutions that positively impact patient care throughout the entire care continuum.
About Robin Pettini

Robin Pettini is the Senior Clinical Marketing Consultant at Siemens-Healthineers Point of Care. She graduated from the University of Massachusetts in 1990 with a B. Sc in Nursing and has continued coursework at Yale University. She has served as adjunct faculty at Capital College in Hartford Connecticut.
Robin has extensive experience in emergency nursing having worked as a Registered Nurse at large level I trauma centers, small community hospitals, and urgent cares centers across the United States. For the last 20 years, she has worked for Point of Care assay manufacturers and has lectured regarding the clinical application of critical laboratory assays both in the United States and globally.