The coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused several waves of infection worldwide. In addition to waves that were caused by the wild-type strain of SARS-CoV-2 several variants of concern (VOCs) have also been responsible for recent spikes in COVID-19 cases. Notably, most SARS-CoV-2 VOCs are associated with a more rapid spread, increased disease severity, or host immune evasion.
SARS-CoV-2 VOCs have been largely responsible for new surges in COVID-19 cases, such as the Alpha variant that showed markedly increased transmissibility, leading to a reproduction number that was 50-100% higher than the earlier SARS-CoV-2 wildtype strain. Notably, the SARS-CoV-2 Alpha strain has 14 non-synonymous mutations in the spike protein, its receptor-binding domain (RBD), and the nucleocapsid (N) genes.
 Study: Waning of the Humoral Response to SARS-CoV-2 in Pregnancy is Variant-Dependent. Image Credit: Marina Demidiuk / Shutterstock.com
Study: Waning of the Humoral Response to SARS-CoV-2 in Pregnancy is Variant-Dependent. Image Credit: Marina Demidiuk / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Almost half of the changes are in the spike gene, which changes the way this protein interacts with the host receptor, the human angiotensin-converting enzyme 2 (hACE2) receptor, thereby resulting in a higher infection rate. The Alpha strain was the first VOC to be identified as such by Public Health England in fall 2020.
The current study from Israel, which appears on the medRxiv* preprint server, discusses the change in antibody response with the trimester of infection and the infecting variant.
Background
In Israel, the Alpha variant triggered the third wave of COVID-19, with 40% higher transmission rates as compared to the wildtype strain of SARS-CoV-2. Simultaneously, the Israeli government was offering the Pfizer BNT162b2 vaccine, which is built on the messenger ribonucleic acid (mRNA) platform, to pregnant women.
Vaccinating pregnant women was largely supported by the need to reduce severe complications of COVID-19 during pregnancy, coupled with indications that this VOC was more dangerous in women than in men. In pregnancy, the antibody response to SARS-CoV-2 appears to be muted.
Earlier studies confirm the safety and benefits of vaccination against COVID-19 in pregnancy, with the immunoglobulin antibody IgG crossing the placenta to protect the fetus and newborn as well. Not much is known about antibody concentrations in the mother and baby after natural infection or vaccination early in pregnancy.
IgM antibodies do not appear to cross the placental barrier. This allows investigators to identify fetal infections as compared to fetal passive immunity. In very few cases, cord blood has shown the presence of IgM antibodies after maternal infection, but not after vaccination, thus indicating that vaccine antigens do not cross the placenta.
The current paper continues with the Israel COVID-19 in Pregnancy study, with a focus on the humoral response and the waning of immunity after either infection or vaccination during pregnancy in order to compare the impact of the Alpha strain to that of the wildtype variant.
Study findings
The study showed that about 17% and 14% of control and vaccinated participants were seropositive for SARS-CoV-2 infection without a prior history. Among the newborn babies in the control group, 3.6% (17) showed a high fetal IgM response, which was compared to six and four newborns from the infected and vaccinated groups, respectively.
Antibody response in pregnancy
Infected cohort
The earlier in pregnancy in which the SARS-CoV-2 infection occurred, the lower were the IgM and IgG concentrations in maternal serum. Especially in the second wave, a history of first- or second-trimester infection was linked to lower maternal IgG antibodies against the S1 subunit of the spike, the RBD, N, and IgM-S1, as well as cord blood IgM-S1, relative to those infected in the third trimester.
With the third wave of infection, maternal IgG against all antigens, as well as IgM against the S2 and N antigens, was much lower following infection during the second trimester as compared to those that occurred in the third trimester. However, antibody titers were lower with second-trimester infections during the third wave as compared to those that occurred in the second wave.
With third trimester infections, IgM antibodies to S1 and the RBD were higher in the second wave than for the third wave; however, cord blood showed the opposite trend for these antibodies.
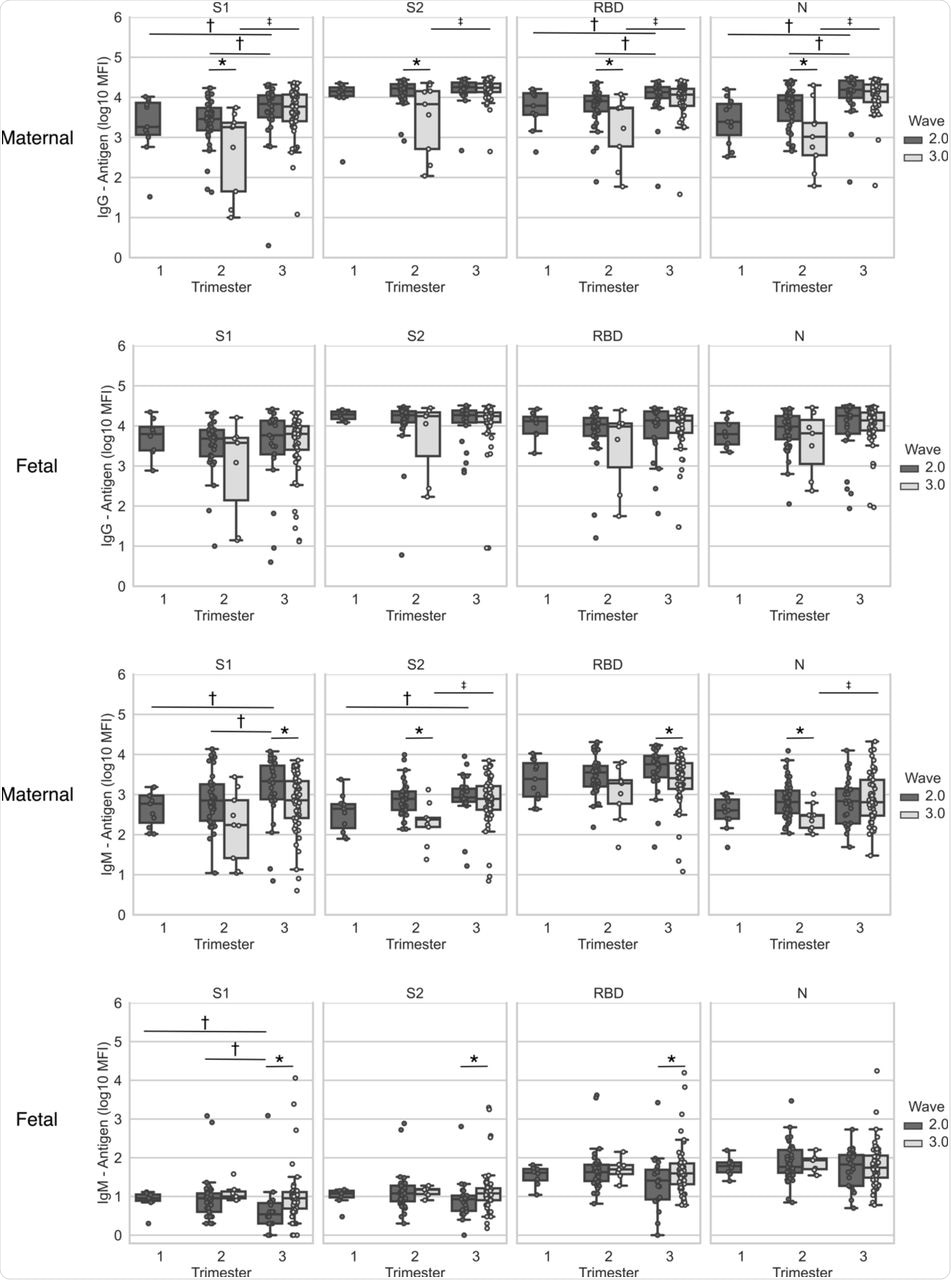 Maternal and cord blood was derived at the time of delivery from patients who recovered from infection during pregnancy with SARS-CoV-2 verified by RT-PCR (n=161). Maternal and fetal IgG and IgM antigens (S1, S2, RBD and N) concentrations are plotted per wave and per trimester of infection. Top two rows, IgG; bottom two rows, IgM. Statistical significance: * indicate significant differences between waves of infection within the same trimester (Wilcoxon Rank Sum Test), † indicate differences between trimesters in the 2nd wave (Kruskall–Wallis one-way ANOVA), and ‡ indicates differences between trimesters in the 3rd wave (Wilcoxon Rank Sum Test). Box and whiskers: Middle line = Median; Box= 25th and 75th percentiles; whiskers= are the minimum (Q1 – 1.5*IQR) and maximum (Q3 + 1.5*IQR).
Maternal and cord blood was derived at the time of delivery from patients who recovered from infection during pregnancy with SARS-CoV-2 verified by RT-PCR (n=161). Maternal and fetal IgG and IgM antigens (S1, S2, RBD and N) concentrations are plotted per wave and per trimester of infection. Top two rows, IgG; bottom two rows, IgM. Statistical significance: * indicate significant differences between waves of infection within the same trimester (Wilcoxon Rank Sum Test), † indicate differences between trimesters in the 2nd wave (Kruskall–Wallis one-way ANOVA), and ‡ indicates differences between trimesters in the 3rd wave (Wilcoxon Rank Sum Test). Box and whiskers: Middle line = Median; Box= 25th and 75th percentiles; whiskers= are the minimum (Q1 – 1.5*IQR) and maximum (Q3 + 1.5*IQR).
Vaccinated cohort
The scientists also found that maternal IgG antibodies to S1 and the RBD, as well as IgM-S1, were higher at the time of childbirth when the vaccine was offered in the third rather than the second trimester. The exposure-delivery interval was analyzed in each case to adjust for the difference in gestational age at delivery.
In this case, antibody titers waned similarly between vaccination or infections and the time of childbirth with second wave infections. For the third wave, the decay of protective antibodies in the mother-child dyad was much faster than with second wave infection or even second wave vaccination.
Maternal to fetal antibody transfer
The current study also shows that second-trimester vaccination or infection during the second wave resulted in a higher rate of antibody transfer to the fetus as compared to the third wave groups. Cord blood antibodies were higher for both second and third wave infections compared to vaccinated or control cohorts.
Conversely, maternal-cord blood antibody ratios were different only between controls and other groups, without any difference by fetal sex.
Cord serum IgM
Of about 475 samples of cord blood, 17 showed a marked rise in IgM antibodies to three or more viral antigens. The presence of these antibodies does not reflect the maternal antibody response.
Implications
The current study findings indicate that the Alpha VOC induced a weaker antibody response in pregnancy compared to the ancestral variant. This is reported here for the first time. Earlier studies indicated that pregnancy increased the risk of severe COVID-19, and infections with the Alpha variant were linked to more adverse outcomes compared to wild-type virus infection.
Simultaneously, the authors of the current study showed lower titers of IgG antibodies to the spike RBD in pregnancy after Alpha VOC infection compared to non-pregnant patients.
The implications of these findings are that the Alpha variant is less immunogenic and is capable of evading host immunity, at least in part, during pregnancy. However, antibody transfer to the fetus did not vary much by the infecting variant, thereby indicating that it is independent of the maternal antibody response and the type of mutations present.
Placental regulation of antibody transport, as well as the presence of active transport, are both shown to be important in this process. The differences in cord blood IgG antibodies to the flu virus between those born to SARS-CoV-2-infected as compared to vaccinated/control mothers seem to show that SARS-CoV-2 infection damages the placenta. This reduces neonatal immunity to the influenza virus.
In fact, more recent studies seem to show such disruption of placental structure. Finally, there were no differences in cord Ig levels by fetal sex.
“The decline of antibody titers following infection attracted much attention because of the emergence of several variants of concern and since it serves as the basis for vaccination policies in previously infected or vaccinated individuals.”
Both infection and vaccination appear to be associated with long-term protective antibody-mediated immunity. At six months from antigen exposure, the blood contained detectable antibody titers. The current study adds to this understanding, showing that antibody titers in pregnancy also wane with the same velocity in vaccinated or infected mothers during the second wave caused mostly by the wild-type variant.
During the third wave, infections led to a rise in antibody titers but then a substantially faster decline. Since the peak antibody titer is itself lower with the Alpha VOC compared to the wild-type virus, these findings indicate a weaker and less durable antibody response to the Alpha variant during pregnancy.
To reduce the clinical risk, pregnant women who were infected before or during early pregnancy, especially with the Alpha variant, should probably receive a booster dose with the wild-type variant. The rapid decrease in antibodies may increase their risk for reinfection otherwise.
The mild fall in antibody titers with the term of pregnancy is not accompanied by similar changes in the cord blood, thus indicating that vaccination efficiently protects both mother and child. The high rate of transfer of IgG antibodies in the women who had first-trimester infections during the Alpha-dominated wave shows that such early infection provides durable fetal immunity.
Fetal IgM has been described in fetal blood from the end of the first trimester, perhaps due to intrauterine infection by antigen exposure from either vaccination or infection; however, actual fetal infection acquired from the mother is exceedingly rare. In this study, 17 newborns showed a strong IgM response, which indicates that the fetus was exposed to viral antigens outside of infection.
Despite the limitations due to the use of antigens from the wildtype strain rather than the Alpha VOC, which could have resulted in altered quantitative results, the current study is the first to examine how the maternal-fetal antibody response changes with time of infection or vaccination, as well as with the infecting strain. This observation demonstrates the variant-dependent nature of the immune response in pregnancy.
It also underlines the role played by placental tissue in regulating antibody transport and mediating active transport of the antibodies to the fetus and its ability to provide fetal immunity after maternal vaccination, thus supporting the important preventive role of COVID-19 vaccination in pregnancy.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.