Vaccination against coronavirus disease 2019 (COVID-19) has been considered to be the most effective way to reduce the mortality and morbidity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Several vaccine candidates have received approval, while many are still at various stages of clinical trials. One of the most potent vaccines that have received Emergency Use Authorization (EUA) from the United States Food and Drug Administration (FDA) is the Pfizer-BioNTech BNT162b2 vaccine.
The BNT162b2 vaccine is a lipid nanoparticle formulation that contains the nucleoside modified messenger ribonucleic acid (mRNA) coding for the SARS-CoV-2 spike glycoprotein. This vaccine was initially approved for administration to individuals who were 16 years of age and above. However, expansion of vaccine eligibility 12 to 15 years old took place in May 2021, with 5 to 11 years approved to receive the vaccine in October 2021.
The results of an ongoing clinical study demonstrated that healthy individuals of 12 years of age or older support the immunogenicity, safety, and efficacy of the 30 micrograms (µg) two-dose BNT162b2 injections that are administered 21 days apart. The vaccine was found to be highly immunogenic in 12 to 15 years old as compared to adults. Moreover, vaccine efficacy in preventing COVID-19 was 95% to 100% from 7 days to 2 months post the second dose.
Although children experience asymptomatic or milder cases of COVID-19 infection, severe illness, as well as long-term complications, can occur after the primary infection. School children played an important role in the transmission of SARS-CoV-2 and thus represented a high proportion of COVID-19 positive cases. COVID-19 related hospitalization was found to increase steadily among children since early July 2021, with a high prevalence among the 5 to 11 years age group in late September 2021.
The COVID-19 pandemic brought about the closure of schools that have impacted the social, emotional, and mental health of children. Therefore, the availability of safe and efficacious vaccines for school children was critical to ensure the opening of the schools while maintaining the safety of the children.
A new study published in The New England Journal of Medicine evaluated the safety, immunogenicity, and efficacy of the BNT162b2 in children who were 5 to 11 years of age.
About the study
The current study recruited children who were 5 to 11 years of age and had no pre-existing medical conditions and no previous COVID-19 diagnosis. Two doses of the BNT162b2 vaccine were administered to these children 21 days apart. Phase I trials comprised an open-label dose-finding study, where three different doses of the vaccine were administered including 10 µg, 20 µg, and 30 µg.
Subsequently, Phase II/III trials consisted of randomized trials where the participants were divided into two groups in a 2:1 ratio to receive either the BNT162b2 vaccine at the assigned dose level or a saline placebo. The reactogenicity data was collected by the parents or guardians for safety evaluations. Blood samples were collected from the children for immunogenicity assessments.
Finally, vaccine efficacy was determined against COVID-19 confirmed cases 7 days post administration of the second dose.
Study findings
Phase I participants were recruited from four sites in the United States. Half of the children were male, 79% were White, 10% were Asian, 8% were Hispanic or Latinx, and 6% were Black. The mean age of the children was found to be 7.9 years.
Phase II/III participants were recruited from 81 sites of the United States along with Spain, Poland, and Finland. Taken together, 52% of these participants were male, 79% White, 6% Asian, 21% Hispanic or Latinx, and 6% were Black. The mean age of the children in this group was found to be 8.2 years.
Phase I safety and immunogenicity study determined that most of the local reactions upon vaccination were mild to moderate and were transient. Fever was more commonly observed in the 30 µg dose-level group. Adverse events were also mostly observed in participants who received two 30 µg doses. Therefore, 10 µg doses were considered safe for further assessment in 5 to 11 years olds.
Phase II/III safety study indicated that local reactions and systemic events were mild to moderate and lasted for 1 or 2 days in the BNT162b2 recipient group. The most common local reaction was injection-site pain, followed by headache and fatigue. Furthermore, systemic events were more commonly reported after the second dose of the vaccine as compared to the first dose.
Although fever occurred in 8.3% of the children after the first or second dose, the use of antipyretics by the recipients was more after the second dose as compared to the first dose. Adverse events were reported by slightly more BNT162b2 recipients as compared to placebo recipients. Also, few BNT162b2 recipients reported the onset of rashes 7 days or more post-vaccination.
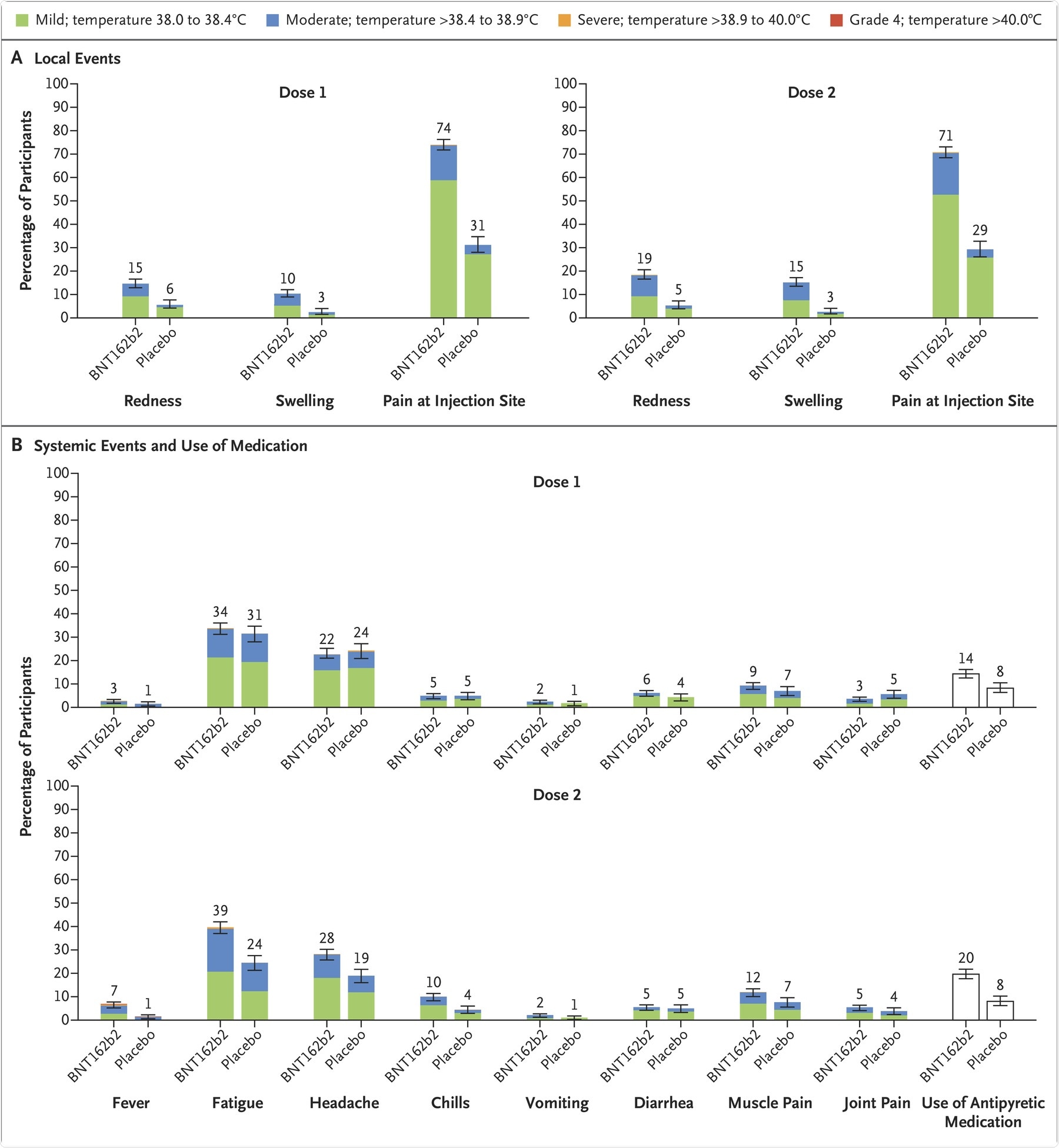 Panel A shows local reactions and Panel B shows systemic events after the first and second doses in recipients of the BNT162b2 vaccine (dose 1, 1511 children; dose 2, 1501 children) and placebo (dose 1, 748 or 749 children; dose 2, 740 or 741 children). The numbers refer to the numbers of children reporting at least one “yes” or “no” response for the specified event after each dose; responses may not have been reported for every type of event. Severity scales are summarized in Table S5; fever categories are designated in the key. The numbers above the bars are the percentage of participants in each group with the specified local reaction or systemic event. Bars represent 95% confidence intervals. One participant in the BNT162b2 group had a fever of 40.0°C after the second dose.
Panel A shows local reactions and Panel B shows systemic events after the first and second doses in recipients of the BNT162b2 vaccine (dose 1, 1511 children; dose 2, 1501 children) and placebo (dose 1, 748 or 749 children; dose 2, 740 or 741 children). The numbers refer to the numbers of children reporting at least one “yes” or “no” response for the specified event after each dose; responses may not have been reported for every type of event. Severity scales are summarized in Table S5; fever categories are designated in the key. The numbers above the bars are the percentage of participants in each group with the specified local reaction or systemic event. Bars represent 95% confidence intervals. One participant in the BNT162b2 group had a fever of 40.0°C after the second dose.
Phase II/III immunogenicity data indicated that the serum neutralizing geometric mean titers (GMT) one month after the second dose of the vaccine was more in the 5 to 11 years group as compared to the 16 to 25 years old group. Furthermore, the Phase II/III efficacy study indicated that vaccine efficacy was 90.7% against COVID-19 infection.
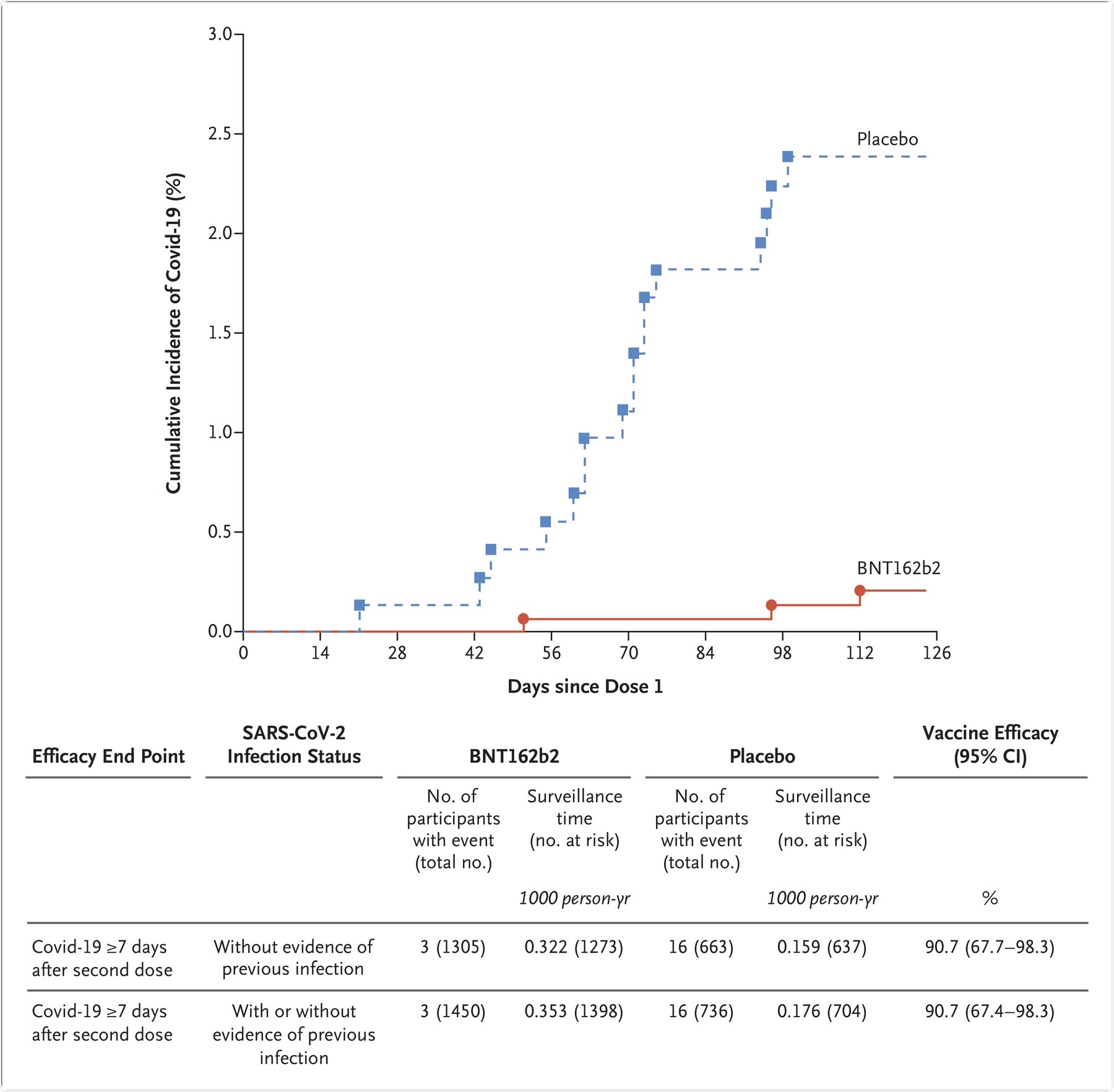 The graph represents the cumulative incidence of the first occurrence of Covid-19 after the first dose of vaccine or placebo. Each symbol represents cases of Covid-19 starting on a given day. Results shown in the graph are all available data for the efficacy population, and the results shown in the table are those for the efficacy population that could be evaluated (defined in Table S1). Participants without evidence of the previous infection were those who had no medical history of Covid-19 and no serologic or virologic evidence of past SARS-CoV-2 infection before 7 days after the second dose (i.e., N-binding serum antibody was negative at the first vaccination visit, SARS-CoV-2 was not detected in nasal swabs by nucleic acid amplification test at the vaccination visits, and nucleic acid amplification tests were negative at any unscheduled visit before 7 days after the second dose). The cutoff date for the efficacy evaluation was October 8, 2021. Surveillance time is the total time in 1000 person-years for the given end point across all participants within each group at risk for the endpoint. The time period for Covid-19 case accrual was from 7 days after the second dose to the end of the surveillance period. The 95% confidence intervals for vaccine efficacy were derived by the Clopper–Pearson method, adjusted for surveillance time.
The graph represents the cumulative incidence of the first occurrence of Covid-19 after the first dose of vaccine or placebo. Each symbol represents cases of Covid-19 starting on a given day. Results shown in the graph are all available data for the efficacy population, and the results shown in the table are those for the efficacy population that could be evaluated (defined in Table S1). Participants without evidence of the previous infection were those who had no medical history of Covid-19 and no serologic or virologic evidence of past SARS-CoV-2 infection before 7 days after the second dose (i.e., N-binding serum antibody was negative at the first vaccination visit, SARS-CoV-2 was not detected in nasal swabs by nucleic acid amplification test at the vaccination visits, and nucleic acid amplification tests were negative at any unscheduled visit before 7 days after the second dose). The cutoff date for the efficacy evaluation was October 8, 2021. Surveillance time is the total time in 1000 person-years for the given end point across all participants within each group at risk for the endpoint. The time period for Covid-19 case accrual was from 7 days after the second dose to the end of the surveillance period. The 95% confidence intervals for vaccine efficacy were derived by the Clopper–Pearson method, adjusted for surveillance time.
Conclusion
Therefore, the current study was quite effective in determining the efficacy, safety, and immunogenicity of an mRNA vaccine administered to the 5 to 11 years old group. This group requires prioritization because they are currently accounting for an increased number of cases and hospitalization.
Without effective vaccines, this group could turn into ongoing reservoirs of infection and become responsible for the emergence of new SARS-CoV-2 variants. Thus, further evaluation of vaccines needs to take place to ensure proper vaccination of younger children.
The current study had certain limitations. First, the study did not involve long-term follow-up for assessment of immune responses and safety of the vaccine. Second, the study could not detect the rare potential side effects of the vaccine. Finally, the size of the population recruited in the study was small.