The coronavirus disease 2019 (COVID-19) pandemic has resulted in devastating effects worldwide and currently, vaccines are the only safeguard. Scientists and pharmaceutical companies have successfully developed several different vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the COVID-19.
Patients treated with immunosuppressive drugs were excluded from the clinical trials for COVID-19 vaccines. Thus, there is not enough evidence on the immune response elicited in these individuals by COVID-19 vaccines.
Immune-mediated inflammatory disorders (IMIDs), such as inflammatory bowel disease (IBD), result from dysregulated immune responses. Biological therapies targeting tumor necrosis factor (TNF) such as infliximab are commonly used to treat IMIDs, including IBD. Vedolizumab is a gut selective anti-integrin a4b7 antibody that is also used to treat IBD.
Previous studies have reported that anti-TNF treated patients showed decreased antibody responses when compared to vedolizumab-treated patients after COVID-19 infection or vaccination with BNT162b2 or ChAdOx1 nCoV-19. A recent study published on the preprint server medRxiv* evaluate the immune responses elicited by the COVID-19 vaccines BNT162b2 or ChAdOx1 nCoV-19 in infliximab- or vedolizumab-treated IBD patients.
Study participants
The present CLARITY study was conducted on 7,226 IBD patients who were recruited between September 22, 2020, and December 23 2020 from 92 hospitals in the United Kingdom. The study population comprised of 2,264 infliximab- and 1,024 vedolizumab-treated patients on continuous therapy with no history of prior SARS-CoV-2 infection. All study participants were subjected to an antibody test 14-70 days subsequent to the second dose of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccines.
The study additionally investigated T-cell response in a subset of participants involving 211 infliximab- and 71 vedolizumab-treated patients and evaluated the effect of the vaccine in 525 infliximab- and 224 vedolizumab-treated participants who had a prior SARS-CoV-2 infection.
Infliximab treatment associated with reduced anti-SARS-CoV-2 spike antibody levels after COVID-19 vaccination
Subsequent to the second dose, the anti-spike (S) receptor-binding domain (RBD) antibody concentrations were found to be higher in recipients of the BNT162b2 vaccine when compared to those who received the ChAdOx1 nCoV-19 vaccine. The reported antibody concentrations for BNT162b2 and ChAdOx1 nCoV-19 were 1,080.2 U/mL and 289.9 U/mL respectively.
After adjusting for confounding factors, such as immunomodulator use, anti-S RBD antibody levels were lower in infliximab-treated patients as compared to the vedolizumab-treated patients in both BNT162b2 (587.9 U/mL vs 4,657.5 U/mL) and ChAdOx1 nCoV-19 vaccine (191.1 U/mL vs 778.6 U/mL) groups.
Multivariable linear regression analysis indicated a four- and six-fold decrease in anti-S RBD levels in the case of vaccinations with BNT162b2 and ChAdOx1 nCoV-19, respectively, when the infliximab group was compared with the vedolizumab group. The analysis also revealed that individuals 60 years or older and patients suffering from Crohn’s disease are associated with lower anti-S RBD antibody levels after receiving either vaccine.
Thiopurine or methotrexate use was associated with low antibody levels in the BNT162b2 vaccine group. Current smoking status, steroid use, and non-white ethnicity were associated with lower antibody levels in ChAdOx1 nCoV-19 vaccine recipients.
Further, vaccination with the BNT162b2 vaccine was associated with 3.7-fold higher antibody concentrations when compared with the ChAdOx1 nCoV-19 vaccine. It was also found that 5.9% of infliximab-treated patients failed to seroconvert compared to 1.3% in the vedolizumab group.
Uncoupling of antibody response and T-cell response after COVID-19 vaccination
When the anti-S T-cell responses were investigated, there were no significant differences between the infliximab-treated or vedolizumab group in the case of both vaccines. Further, one-fifth of the participants did not exhibit T-cell responses.
Even in the absence of an antibody response after the first COVID-19 vaccine dose, T-cell responses were detected, thus demonstrating the uncoupling of T-cell responses and the antibody response. A small percentage of participants, 4.5 % in the case of BNT162b2 and 1.8% in the case of ChAdOx1 nCoV-19, did not show both antibody or T-cell responses after the second dose.
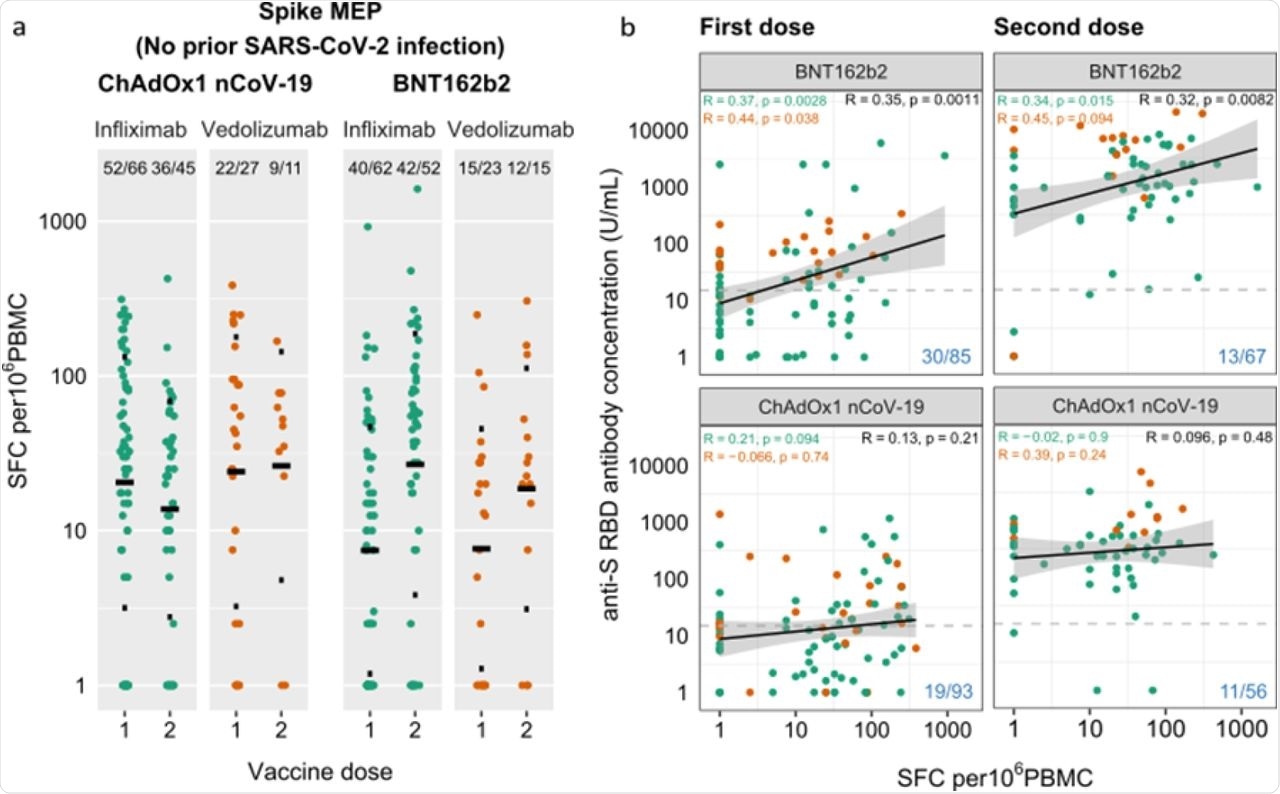
 Anti-SARS-CoV-2 spike T cell responses stratified by vaccine platform (BNT162b2 vs ChAdOx1 nCoV-19), biologic therapy (infliximab vs vedolizumab), and vaccine dose (one vs two). a. Spike MEP T cell responses SFC per 106 PBMC stratified by vaccine platform, biologic therapy (infliximab vs vedolizumab) and number of vaccine doses. The horizontal bar represents the geometric mean and the narrow bars represent one geometric standard deviation either side of the geometric mean. The number of T cell responders / total number of individuals tested are shown in black at the top of each panel. b. Scatterplot demonstrating the correlation between T cell responses against spike MEP pool (SFC per 106 PBMC) and anti-SARS-CoV-2 spike antibody concentration after the first (LHS) and second (RHS) dose of BNT162B2 (top) and ChAdOx1 nCoV-19 (bottom) vaccine. The number of non-T cell responders / total number of individuals tested is shown in blue on the bottom RHS of each panel. The horizontal dotted line in b. represents a threshold of 15 U/mL of anti-S1 SARS-CoV-2 antibody. The biologic infliximab is show in green and vedolizumab is shown in orange. R, Spearman’s rank correlation. SFC, spot forming cells. PBMC, peripheral blood mononuclear cell. MEP, mapped epitope peptide.
Anti-SARS-CoV-2 spike T cell responses stratified by vaccine platform (BNT162b2 vs ChAdOx1 nCoV-19), biologic therapy (infliximab vs vedolizumab), and vaccine dose (one vs two). a. Spike MEP T cell responses SFC per 106 PBMC stratified by vaccine platform, biologic therapy (infliximab vs vedolizumab) and number of vaccine doses. The horizontal bar represents the geometric mean and the narrow bars represent one geometric standard deviation either side of the geometric mean. The number of T cell responders / total number of individuals tested are shown in black at the top of each panel. b. Scatterplot demonstrating the correlation between T cell responses against spike MEP pool (SFC per 106 PBMC) and anti-SARS-CoV-2 spike antibody concentration after the first (LHS) and second (RHS) dose of BNT162B2 (top) and ChAdOx1 nCoV-19 (bottom) vaccine. The number of non-T cell responders / total number of individuals tested is shown in blue on the bottom RHS of each panel. The horizontal dotted line in b. represents a threshold of 15 U/mL of anti-S1 SARS-CoV-2 antibody. The biologic infliximab is show in green and vedolizumab is shown in orange. R, Spearman’s rank correlation. SFC, spot forming cells. PBMC, peripheral blood mononuclear cell. MEP, mapped epitope peptide.
The durability of antibody response after COVID-19 vaccination was shorter in infliximab-treated IBD patients
The durability of the antibody response was evaluated. To this end, it was observed that the half-life of anti-S-RBD antibodies elicited by BNT162b2 was 4.6 weeks, which was shorter than that observed for ChAdOx1 nCoV-19, which was 5.9 weeks.
Further, the half-life of anti-S-RBD antibodies in patients treated with infliximab was shorter when compared to the vedolizumab-treated group after the second dose of both vaccines. In patients who received the BNT162b2 vaccine, the half-life of anti-S-RBD antibodies was four weeks for the infliximab-treated group and 7.2 weeks for the vedolizumab-treated group. Comparatively, the half-life of these antibodies in individuals who received the ChAdOx1 nCoV-19 vaccine was 5.3 weeks and 9.3 weeks, respectively.
Seroconversion is the point when antibodies against a virus can be detected in the blood after a viral infection. A more rapid reduction in anti-S RBD concentrations below seroconversion threshold levels was associated with infliximab treatment, current smoking status, and white ethnicity.
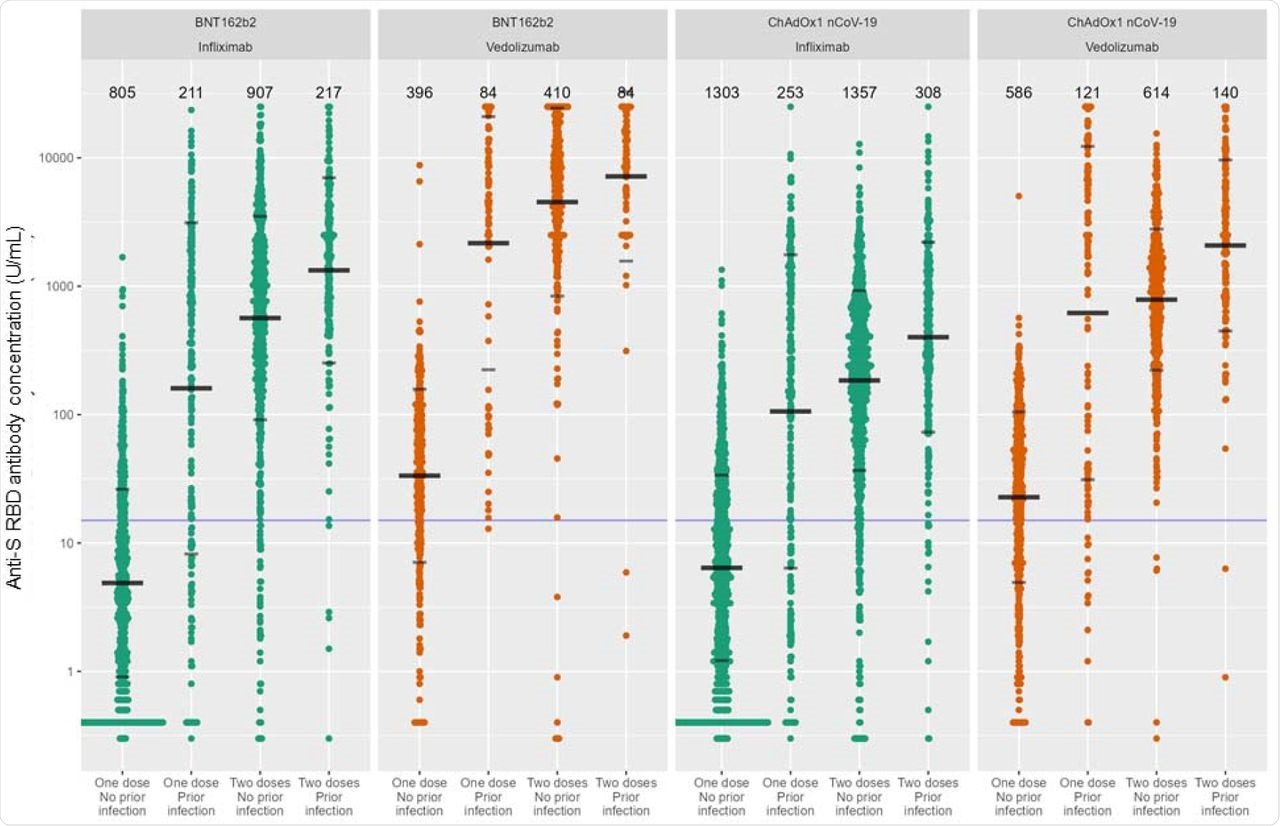
 Anti-S RBD antibody concentration stratified by biologic therapy (infliximab vs vedolizumab), type of vaccine, vaccine dose and history of prior SARS-CoV-2 infection. The wider bar represents the geometric mean, while the narrower bars are drawn one geometric standard deviation either side of the geometric mean. Based on published data using neutralization assays threshold shown of 15 U/mL was used to determine seroconversion11. The biologic treatment infliximab is shown in green and vedolizumab in orange. The number of individuals tested for each group are shown in black at the top of each panel.
Anti-S RBD antibody concentration stratified by biologic therapy (infliximab vs vedolizumab), type of vaccine, vaccine dose and history of prior SARS-CoV-2 infection. The wider bar represents the geometric mean, while the narrower bars are drawn one geometric standard deviation either side of the geometric mean. Based on published data using neutralization assays threshold shown of 15 U/mL was used to determine seroconversion11. The biologic treatment infliximab is shown in green and vedolizumab in orange. The number of individuals tested for each group are shown in black at the top of each panel.
Infliximab-treated IBD patients had higher episodes of breakthrough infections after CVOID-19 vaccination
Of the 5,123 participants in the study, 267 exhibited a positive test for SARS-CoV-2 two or three weeks after receiving the second vaccine dose. None of these participants had a previous positive polymerase chain reaction (PCR) test for SARS-CoV-2 or showed any serological evidence for prior infections.
It was found that 5.8% of participants in the infliximab-treated group developed breakthrough infections compared to 3.9% in the vedolizumab-treated group, This observation indicates that the occurrences of breakthrough infections were higher in participants treated with infliximab.
Infliximab treatment and ChAdOx1 nCoV-19 vaccine were found to be associated with a shorter period to breakthrough infections. In participants who had a breakthrough infection, the levels of anti-S RBD antibodies measured two to ten weeks after the second dose of the vaccines were found to be low.
For a 10-fold increase in anti-S RBD antibody levels, a 0.8-fold reduction in the odds for developing a breakthrough infection was observed. This indicates that the risk for developing breakthrough infections can be predicted by the antibody levels observed after the second dose of COVID-19 vaccines.
Higher anti-S RBD levels and sustained antibody responses observed in patients with prior SARS-CoV-2 infection after COVID-19 vaccinations
The current study also investigated the effect of infliximab and vedolizumab treatment on COVID-19 vaccinations in participants who had a prior history of SARS-CoV-2 infection before vaccination. In the infliximab-treated group, anti-S RBD antibody concentrations after the second dose of BNT162b2 vaccine was 13,330.0 U/mL and after the second dose of ChAdOx1 nCoV-19 was 401.2 U/mL.
In the case of the vedolizumab-treated group, the antibody concentrations after the second dose of BNT162b2 vaccine was 7,169.5 U/mL and 2,077.3 U/mL after the second dose of the ChAdOx1 nCoV-19 vaccine. This indicates lower anti-S RBD antibody concentrations in the infliximab-treated group compared to the vedolizumab group for both vaccines.
Notably, sustained antibody responses were observed in patients who had SARS-CoV-2 infection before vaccination. This was not influenced by whether the patients were treated with infliximab or vedolizumab.
In patients with previous SARS-CoV-2 infection, a second dose of a COVID-19 vaccine will be a third antigenic exposure, which has been found to result in a sustained antibody response. This suggests that a third dose of the COVID-19 vaccine may benefit certain patient populations who have an increased risk of developing COVID-19.
Conclusion
Patients suffering from IBD and treated with infliximab were found to show reduced antibody levels, less durable anti-S RBD antibody responses, and a higher incidence of breakthrough infections after receiving the COVID-19 vaccines BNT162b2 or ChAdOx1 nCoV-19. BNT162b2 vaccination resulted in higher peak antibody levels and lower incidence of breakthrough infections in infliximab-treated individuals when compared to ChAdOx1 nCoV-19. Further, the third dose of COVID-19 vaccines may be recommended for patients treated with anti-TNF therapy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lin, S., Kennedy, N. A., Saifuddin, A., et al. (2021). Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in infliximab- and vedolizumab-treated patients. medRxiv. doi:10.1101/2021.11.10.21266168. http://medrxiv.org/lookup/doi/10.1101/2021.11.10.21266168.
- Peer reviewed and published scientific report.
Lin, Simeng, Nicholas A. Kennedy, Aamir Saifuddin, Diana Muñoz Sandoval, Catherine J. Reynolds, Rocio Castro Seoane, Sherine H. Kottoor, et al. 2022. “Antibody Decay, T Cell Immunity and Breakthrough Infections Following Two SARS-CoV-2 Vaccine Doses in Inflammatory Bowel Disease Patients Treated with Infliximab and Vedolizumab.” Nature Communications 13 (1). https://doi.org/10.1038/s41467-022-28517-z. https://www.nature.com/articles/s41467-022-28517-z.