A team of scientists from Germany has recently conducted a study to investigate the effect of inhaled prostacyclin on the prognosis of acute respiratory distress syndrome (ARDS) induced by coronavirus disease 2019 (COVID-19).
The findings reveal that inhaled prostacyclin significantly improves oxygenation in ARDS patients who had COVID-19. The study is currently available on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Acute respiratory distress syndrome (ARDS), characterized by severe hypoxia, is a life-threatening condition. Severe hypoxia developed in COVID-19 patients is also associated with ARDS. Long-lasting hyperinflammation is the main causative factor for ARDS, affecting alveolar barrier function. In its severe form, ARDS can lead to hypoxia-induced organ failure.
Inhaled prostacyclin is commonly used to treat changes in pulmonary vasculature observed in patients with dyspnea. Prostacyclin-based therapies are expected to be beneficial in COVID-19 patients as the disease is associated with endothelial injury.
In the current study, the scientists have assessed the effect of inhaled prostacyclin on oxygenation level, organ failure, disease severity, and mortality in ARDS patients with or without COVID-19.
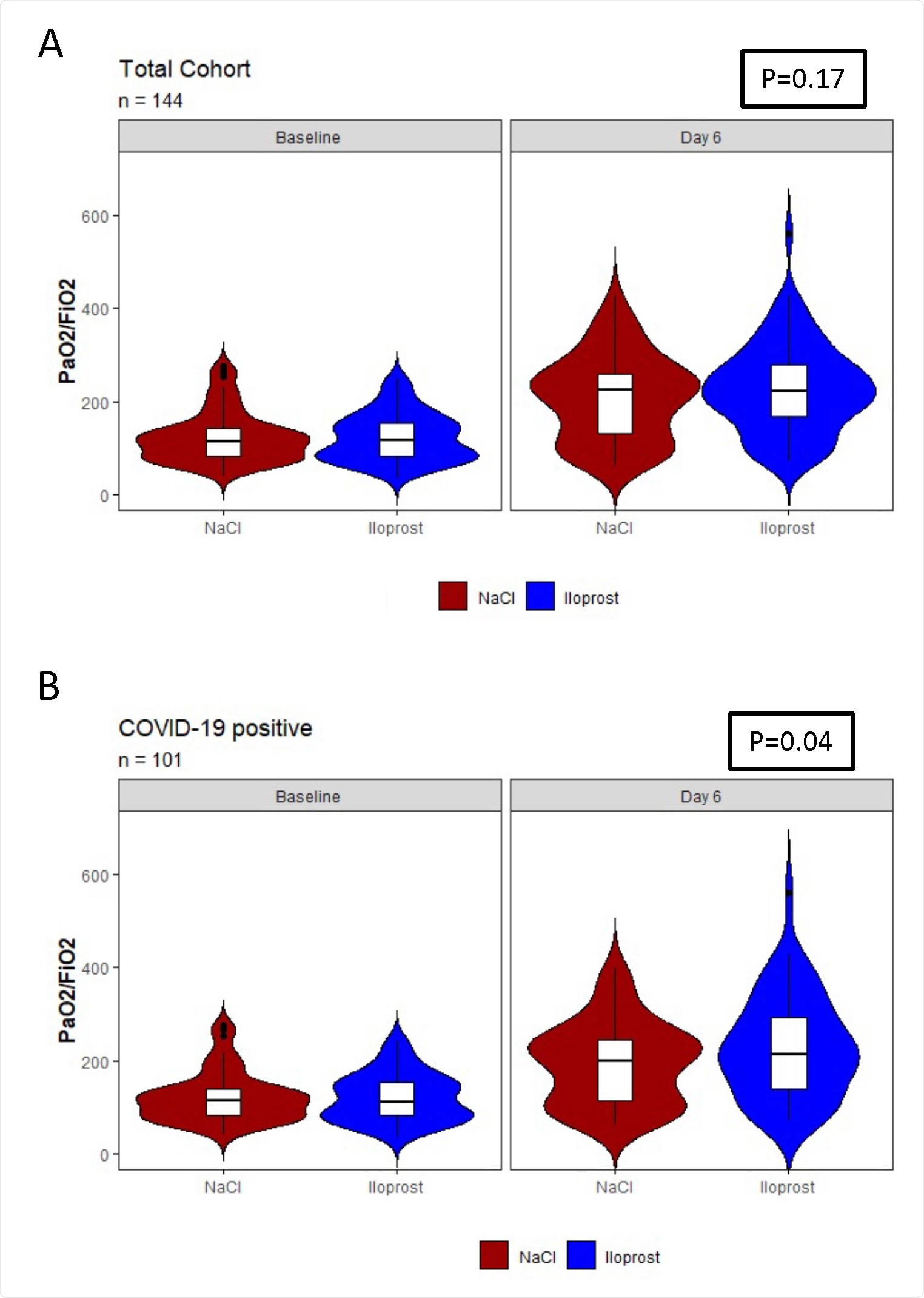
Oxygenation on Day 5 of treatment (day 6 following baseline) in the prostacyclin-treated group compared the control (NaCl)-treated group among A) all patients included in the trial and B) all COVID-19+ patients included in the trial.
Study design
The study was conducted on moderate to severe ARDS patients between March 2019 and August 2021. A total of 144 patients were randomly categorized into two groups. The treatment group received inhaled prostacyclin three times a day for five days. The control group patients received sodium chloride.
Upon completion of the therapy, study parameters including oxygenation index, mortality, organ failure, disease severity, and adverse events were assessed during the 90-day follow-up period.
For disease severity and adversity assessment, duration of ICU stay/mechanical ventilation, ventilation-related pneumonia, pulmonary/gastrointestinal hemorrhage, pulmonary embolism, coagulopathy, and delirium was analyzed.
Important observations
The patients from both treatment and control groups shared similar baseline characteristics. However, a higher incidence of preexisting emphysema and chronic obstructive pulmonary disease (COPD) was observed in patients in the treatment group. The leading cause of ARDS onset was identified as COVID-19, followed by bacterial infection.
Oxygenation index
No significant difference in oxygenation index, defined as the improvement in oxygenation level, was observed between the two groups. Considering all patients with or without COVID-19, the oxygenation index showed a tendency to improve following prostacyclin treatment. Specifically, prostacyclin treatment caused an improvement in oxygenation by 22 mmHg as compared to sodium chloride treatment. However, no significant impact of age, sex, injury type, or COVID-19 status on oxygenation index was observed in ARDS patients.
When considering patients with COVID-19-induced ARDS, a significant improvement in oxygenation index by 34 mmHg was observed after prostacyclin treatment. The treatment effect was relatively higher in patients aged 70 years or above. Similarly, a higher effect of treatment was observed in patients with direct injury compared to those with indirect injury.
Disease severity and mortality
No significant difference in mortality rate, organ failure incidence, and disease severity was observed between the treatment and control groups. Furthermore, when considering patients with COVID-19-induced ARDS, no effect of prostacyclin treatment on these secondary parameters was observed.
Adverse events
No significant benefit of prostacyclin treatment on ARDS-related adversities was observed in study patients. Both sodium chloride-treated and prostacyclin-treated patients showed similar incidence of bleeding problems, pulmonary embolism, coagulopathy, and gastrointestinal, cardiovascular, and neurological complications. The requirement for transfusion and renal replacement therapy also remained similar in the two groups.
When considering patients with COVID-19-induced ARDS, no difference in the incidence of adverse events was observed between the groups.
Study significance
The study highlights the benefits of inhaled prostacyclin in improving oxygenation among patients with ARDS. The treatment effect is more pronounced in patients who have developed ARDS due to COVID-19. However, despite improved oxygenation, no additional benefits of prostacyclin on disease severity, adverse events, and mortality have been observed in ARDS patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Haeberle H. 2021. Inhaled Prostacyclin Improves Oxygenation in Patients with COVID-19-induced Acute Respiratory Distress Syndrome, medRxiv, https://www.medrxiv.org/content/10.1101/2021.11.15.21266343v1
- Peer reviewed and published scientific report.
Haeberle, Helene A., Stefanie Calov, Peter Martus, Lina Maria Serna-Higuita, Michael Koeppen, Almuth Goll, Alice Bernard, et al. 2023. “Inhaled Prostacyclin Therapy in the Acute Respiratory Distress Syndrome: A Randomized Controlled Multicenter Trial.” Respiratory Research 24 (1). https://doi.org/10.1186/s12931-023-02346-0. https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-023-02346-0.