Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus, emerged in Wuhan, China, in late 2019 and has become a significant challenge to human health.
The virus causes COVID-19 with mild to severe symptoms, with some people experiencing acute respiratory distress syndrome (ARDS), organ failure, and risk of death. The SARS-CoV-2 infection causes immune dysregulation and hyperactivation, characterized by the overproduction of cytokines leading to a “cytokine storm”, which causes systemic hyper-inflammation.
Although many studies have focused on the role of cytokines and related cellular responses, not much is known about the role of innate immunity and its contribution to host responses and COVID-19 progression.
Analyzing the role of NK and T cell subsets in SARS-CoV-2 infection
Although natural killer (NK) and T cells play a key role in COVID-19 progression by stimulating cytokine release, their role in clinical SARS-CoV-2 infection has not been studied well.
A new study published in the open-access journal Cells aimed to clarify the role of NK and T cell subsets in SARS-CoV-2 infection in hospitalized COVID-19 patients to unravel their potential association with disease severity.
The researchers enrolled 35 hospitalized COVID-19 patients with a confirmed viral RNA-positive nasopharyngeal swab and 17 healthy sex- and age-matched controls. Demographic and data, radiological and immunological features of the participants, and their serum concentrations of inflammatory biomarkers were recorded in an electronic database.
The study cohort was stratified into mild, moderate, and severe COVID-19 subgroups. The mild subgroup needed pharmacological treatment and low-flow oxygen; the moderate subgroup needed non-invasive mechanical ventilation and/or high-flow oxygen; the severe subgroup patients were admitted to intensive care units and needed mechanical ventilation with orotracheal intubation.
A key inclusion criterion for the study participants was documented monolateral or bilateral pneumonia using chest X-ray or high-resolution computed tomography (HRCT). Blood samples were collected for immunological studies within 24–48 h of admission to the emergency room and before administration of any steroids, antiviral agents, and/or immunosuppressants. NK cell and T cell subsets were analyzed using flow cytometry and serum cytokines were detected using a bead-based multiplex assay.
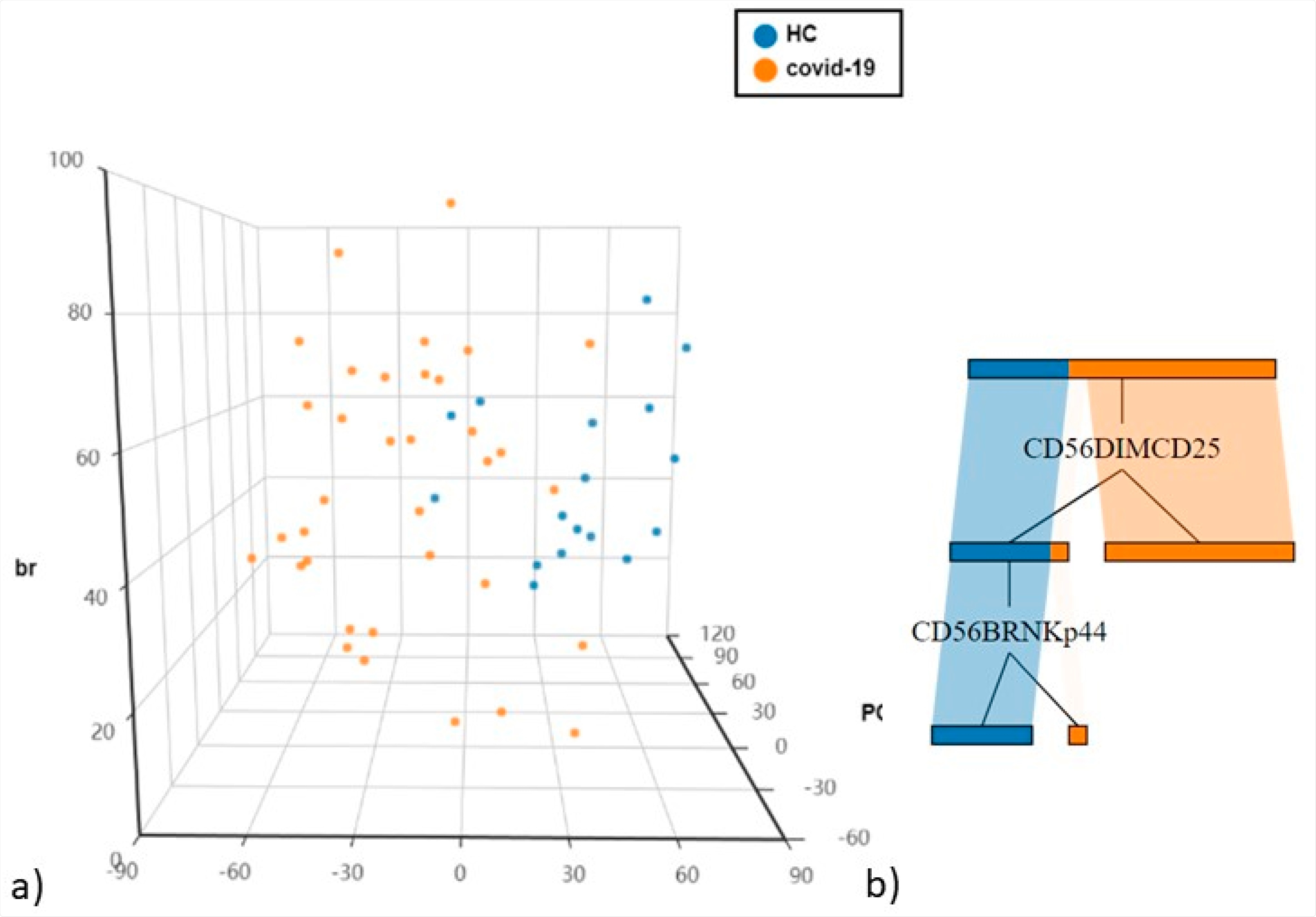
(a) Principal Component Analysis (PCA) of NK cells and T cell subsets (b) The cellular subsets of patients were employed to create a decision tree model for the detection of best clustering variables between the two groups.
Results show significantly higher NK cell frequency in severe COVID-19 patients
The results observed fewer CD56dimCD16brightNKG2A+NK cells and a parallel increase in the CD56+CD69+NK, CD56+PD-1+NK, CD56+NKp44+NK subsets in COVID-19 patients than in the healthy controls.
The study also reported a significantly higher adaptive/memory-like NK cell frequency in patients with severe COVID-19 than in those with mild and moderate phenotypes. Also, adaptive/memory-like NK cell frequencies were significantly higher in COVID-19 patients who died than in COVID-19 survivors.
Patients with severe COVID-19 showed higher serum IL-6 concentrations than those with mild COVID-19 and healthy controls. A direct correlation was noted between IL-6 and adaptive/memory-like NK.
Another crucial observation is the increased expression of programmed death-1 (PD-1) in CD56dimNK cells. PD-1 is an immunoregulatory receptor expressed by various immune cell subtypes such as NK cells and T cells.
With respect to comorbidities, evidence shows that immunometobolic dysregulation exaggerated by obesity and viral infection can impact COVID-19 progression leading to a severe disease course. Although the controls in this study were sex-matched, they did not have any chronic diseases or comorbidities which can be deemed as a bias of the study.
Lymphocyte subset surveillance may help manage COVID-19 patients
Taken together, these findings offer valuable insights into the immune response of COVID-19 patients. They demonstrate NK activation through overexpression of CD69 and CD25 and show that PD-1 inhibitory signaling helps maintain an exhausted phenotype in NK cells.
For T cells, CD8 plays a critical role, which demonstrates a lower proportion in severe COVID-19 patients than seen in mild to moderate COVID-19 patients.
The dysregulation of immune responses in these patients suggests that surveillance of lymphocyte subsets may be helpful in the clinical management of patients with COVID-19. These observations also agree with the immunological changes recently described in a hospitalized, mild-to-moderate COVID-19 patient who showed a significant spike in activated T cells. These findings suggest that adaptive/memory-like NK cells could be the basis of an effective, targeted antiviral therapy for future infections.