Asthma is among the most prevalent chronic inflammatory lung diseases that affects over 5% of the global population. The main causative agents of asthma onset, exacerbation, and progression include rhinoviruses (RV) and inhaled allergens like house dust mites (HDM). Host cells recognize the ribonucleic acid (RNA) of RV using the endosomal toll-like receptor 3 (TLR) 3, TLR7/8, and cytoplasmic RNA helicases: retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5).
Severe acute respiratory syndrome coronavirus (SARS-CoV-2), the novel coronavirus responsible for the coronavirus disease 2019 (COVID-19) pandemic, has been shown to cause respiratory illness, however, its role in asthma exacerbations is poorly understood. It is also not clear if preexisting asthma is a risk factor for SARS-CoV-2 infection or severe disease or if it offers protection from the disease.
In a recent study, researchers explored the effects of RV, HDM, and SARS-CoV-2 on differentiated primary human bronchial epithelial cells (HBECs) in vitro and in experimental in vivo RV infection in healthy subjects and asthmatic patients.
 Study: Epithelial RIG-I inflammasome activation suppresses antiviral immunity and promotes inflammatory responses in virus-induced asthma exacerbations and COVID-19. Image Credit: Antonio Guillem / Shutterstock
Study: Epithelial RIG-I inflammasome activation suppresses antiviral immunity and promotes inflammatory responses in virus-induced asthma exacerbations and COVID-19. Image Credit: Antonio Guillem / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Investigations and outcomes
In this pre-print research paper posted to the medRxiv* server, the researchers discussed their investigations of the release of mature Interleukin 1 beta (IL-1b) in differentiated primary human bronchial epithelial cells (HBECs) upon rhinovirus A16 (RV-A16) infection with and without exposure to HDM in a dose and time-dependent manner and noted that there was a formation and activation of inflammasome in the differentiated primary HBECs. Also, the activated inflammasome was enhanced by the exposure to HDM, especially in asthmatic patients with earlier higher pro-IL-1b expression at baseline.
The researchers also found that the pattern recognition receptors (PRR) expressed in the human epithelium acts as a sensor and activator of inflammasome assembly. It was observed that RIG-I inflammasome activation was strongly enhanced in the epithelium of asthmatic patients, primarily in the presence of HDM exposure. However, NLRP3 and MDA5 inflammasomes did not play any role.
The researchers investigated the inflammasome- and IL-1b-mediated immune responses to evaluate the effect of enhanced epithelial RIG-I inflammasome activation on the overall inflammatory responses at the bronchus barrier sites in asthma. The study demonstrated that upon RIG-I inflammasome activation, the immune responses by both inflammasomes- and IL-1b were enhanced in both asthmatic and control patients.
The researchers compared the samples from in vivo RV-A16 infections in asthmatic and control individuals, taken two weeks before infection and four days after infection. It was observed that after rhinovirus infection in vivo, there was a sustained bronchial RIG-I inflammasome activation and inflammasome-mediated immune responses in asthma.
The researchers analyzed the status of antiviral genes and proteins involved with in vivo and in vitro responses to RV-A16 infection. The result depicted rapid virus clearance from healthy individuals; however, it was delayed in patients with asthma which may be due to the decrease in the effectiveness of RIG-I-induced antiviral mechanisms.
They further studied the impact of activation of the RIG-I inflammasome on RIG-I dependent interferon signaling in bronchial epithelium of patients with asthma and found that RIG-I dependent interferon signaling was impaired. Therefore, it can be concluded that viral load can be decreased by timely blocking of the excessive RIG-I inflammasome activation and IL-1b signaling.
Their analysis on the implications of HDM pre-exposure on the timing and strength of antiviral responses suggested that HDM impaired interferon responses in rhinovirus-infected bronchial epithelium of patients with asthma. Lastly, the researchers invest
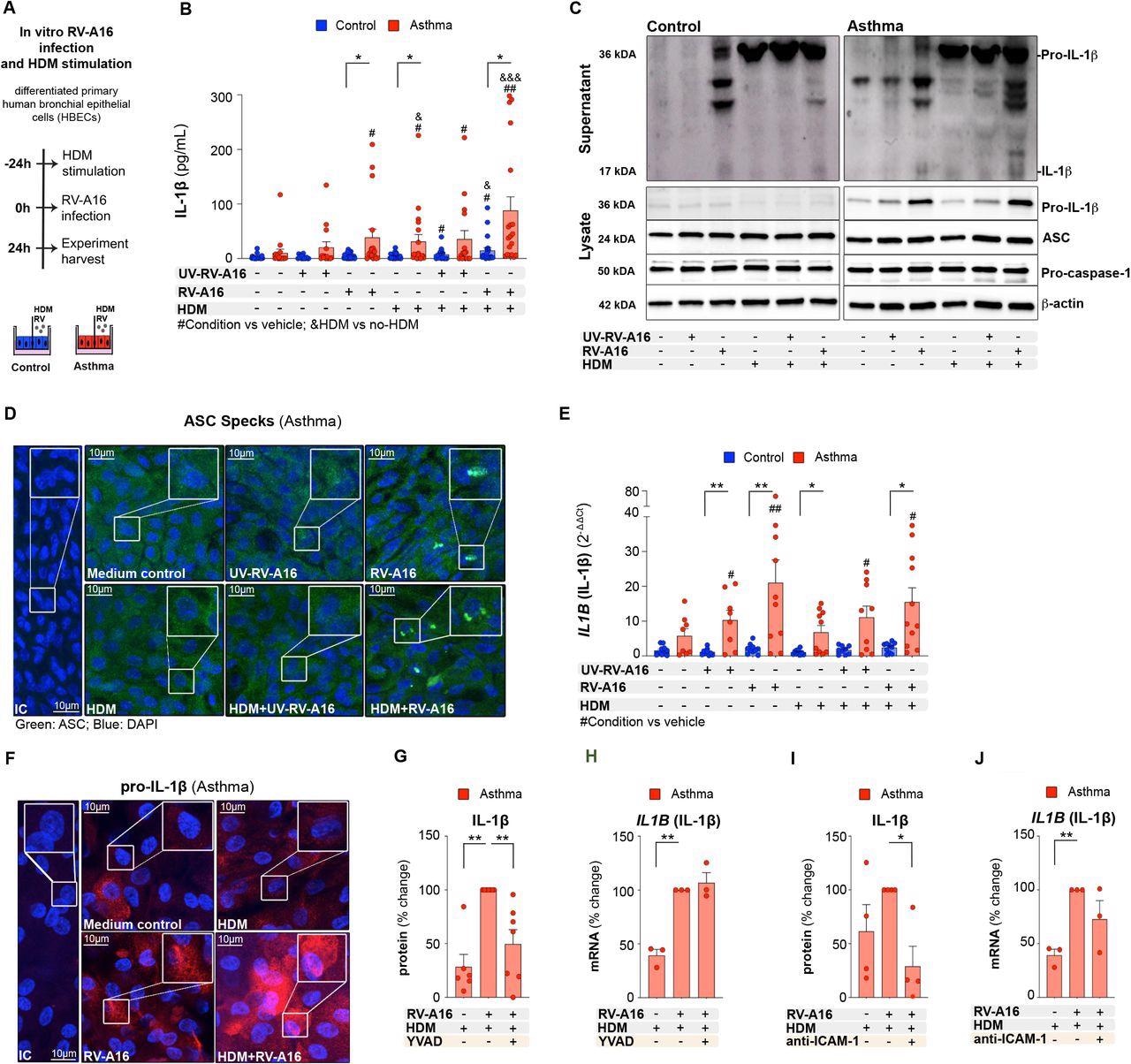 House dust mite increased rhinovirus-induced inflammasome activation in human bronchial epithelium in asthma A) Experimental model overview. Primary air liquid interface (ALI)-differentiated human bronchial epithelial cells (HBECs) from control individuals and patients with asthma were treated with house dust mite (HDM) (200 μg/mL) or vehicle for 24h, followed by an infection with rhinovirus A16 (RV-A16) or UV-treated (UV)-RV-A16 in the multiplicity of infection (MOI) 0.1 for 24h. B) IL-1β release to the apical compartment assessed by ELISA (control n=14-22; asthma n=14-17). C) Representative Western Blot images of secreted IL-1β (apical compartment), and pro-IL-1β, ASC, pro-caspase-1 and β-actin (cell lysates) from HBECs from control subjects (left panel) and patients with asthma (right panel) D) Representative confocal images of ASC speck formation in HBECs from patients with asthma (n=3); scale bars: 10μm. E) mRNA expression of IL1B (IL1β) was assessed using RT-PCR, and presented as a relative quantification (RQ=2-ΔΔCt) compared to the vehicle from the controls (control n=9-10, asthma n=8-10). F) Representative confocal images of IL-1β in HBECs from patients with asthma (n=3); scale bars: 10μm G) IL-1β release to the apical compartment assessed by ELISA (n=6-7), and H) mRNA expression of IL1B (IL1β) (n=3), in HBECs from patients with asthma in the presence or absence of caspase-1 inhibitor (YVAD). Data are presented as the percentage of the response after HDM+RV-A16 treatment. I) IL-1β release to the apical supernatants assessed by ELISA (n=4), and J) mRNA expression of IL1B (IL1β) in HBECs (n=3) from patients with asthma in the presence or absence of anti-ICAM-1 combined with HDM+RV-A16 stimulation. Data are presented as the percentage of the response after HDM+RV-A16 treatment. HBECs from patients with asthma are presented in red, HBECs from control individuals are presented in blue. (*) represents a significant difference as indicated. (#) represents a significant difference of the designated condition as compared to the vehicle from the same group. (&) represents a significant difference upon HDM treatment as compared to the corresponding condition without HDM. Bar graph data show mean ± SEM analyzed with one-way ANOVA (Kruskal-Wallis test), RM one-way ANOVA (Friedman test) or mixed-effects model, as appropriate, depending on the data relation (paired or unpaired) and distribution, *p-value≤0.05, **p-value≤0.01, ***p-value≤0.001. ALI; Air-liquid interface cultures; anti-ICAM-1, anti-ICAM-1 antibody; IC; Isotype control; HDM, house dust mite; RV-A16, rhinovirus A16; UV-RV-A16, UV-treated rhinovirus A16; YVAD, ac-YVAD-cmk (caspase-1 inhibitor); MOI, multiplicity of infection.
House dust mite increased rhinovirus-induced inflammasome activation in human bronchial epithelium in asthma A) Experimental model overview. Primary air liquid interface (ALI)-differentiated human bronchial epithelial cells (HBECs) from control individuals and patients with asthma were treated with house dust mite (HDM) (200 μg/mL) or vehicle for 24h, followed by an infection with rhinovirus A16 (RV-A16) or UV-treated (UV)-RV-A16 in the multiplicity of infection (MOI) 0.1 for 24h. B) IL-1β release to the apical compartment assessed by ELISA (control n=14-22; asthma n=14-17). C) Representative Western Blot images of secreted IL-1β (apical compartment), and pro-IL-1β, ASC, pro-caspase-1 and β-actin (cell lysates) from HBECs from control subjects (left panel) and patients with asthma (right panel) D) Representative confocal images of ASC speck formation in HBECs from patients with asthma (n=3); scale bars: 10μm. E) mRNA expression of IL1B (IL1β) was assessed using RT-PCR, and presented as a relative quantification (RQ=2-ΔΔCt) compared to the vehicle from the controls (control n=9-10, asthma n=8-10). F) Representative confocal images of IL-1β in HBECs from patients with asthma (n=3); scale bars: 10μm G) IL-1β release to the apical compartment assessed by ELISA (n=6-7), and H) mRNA expression of IL1B (IL1β) (n=3), in HBECs from patients with asthma in the presence or absence of caspase-1 inhibitor (YVAD). Data are presented as the percentage of the response after HDM+RV-A16 treatment. I) IL-1β release to the apical supernatants assessed by ELISA (n=4), and J) mRNA expression of IL1B (IL1β) in HBECs (n=3) from patients with asthma in the presence or absence of anti-ICAM-1 combined with HDM+RV-A16 stimulation. Data are presented as the percentage of the response after HDM+RV-A16 treatment. HBECs from patients with asthma are presented in red, HBECs from control individuals are presented in blue. (*) represents a significant difference as indicated. (#) represents a significant difference of the designated condition as compared to the vehicle from the same group. (&) represents a significant difference upon HDM treatment as compared to the corresponding condition without HDM. Bar graph data show mean ± SEM analyzed with one-way ANOVA (Kruskal-Wallis test), RM one-way ANOVA (Friedman test) or mixed-effects model, as appropriate, depending on the data relation (paired or unpaired) and distribution, *p-value≤0.05, **p-value≤0.01, ***p-value≤0.001. ALI; Air-liquid interface cultures; anti-ICAM-1, anti-ICAM-1 antibody; IC; Isotype control; HDM, house dust mite; RV-A16, rhinovirus A16; UV-RV-A16, UV-treated rhinovirus A16; YVAD, ac-YVAD-cmk (caspase-1 inhibitor); MOI, multiplicity of infection.
igated the effect on SARS-CoV-2 infection due to RV-A16-induced RIG-I inflammasome activation and HDM-mediated decrease of IFN responses. It was found that preexisting RV-A16 infection attenuated SARS-CoV-2 replication in asthma.
Conclusion
The study's findings suggested that RV infection and replication activated the RIG-I, but not the NLRP3 inflammasome; RIG-I was further amplified in the presence of HDM. It was also observed that SARS-CoV-2 infection decreased in asthma patients with preexisting RV infection and induction of IFNs in the epithelium, but the RIG-I inflammasome activation and release of proinflammatory mediators increased in the presence of both RV and HDM.
"Timely inhibition of the epithelial RIG-I inflammasome and reduction of IL-1b signaling may lead to more efficient viral clearance and lower the burden of RV and SARS-CoV-2 infection."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Epithelial RIG-I inflammasome activation suppresses antiviral immunity and promotes inflammatory responses in virus-induced asthma exacerbations and COVID-19. U Radzikowska, A Eljaszewicz, G Tan, N Stocker, A Heider, P Westermann, S Steiner, A Dreher, P Wawrzyniak, B Rückert, J Rodriguez-Coira, D Zhakparov, M Huang, B Jakiela, M Sanak, M Moniuszko, L O’Mahony, T Kebadze, DJ Jackson, MR Edwards, V Thiel, SL Johnston, CA Akdis, M Sokolowska, medRxiv, 2021; Doi: https://doi.org/10.1101/2021.11.16.21266115, https://www.medrxiv.org/content/10.1101/2021.11.16.21266115v1
- Peer reviewed and published scientific report.
Radzikowska, Urszula, Andrzej Eljaszewicz, Ge Tan, Nino Stocker, Anja Heider, Patrick Westermann, Silvio Steiner, et al. 2023. “Rhinovirus-Induced Epithelial RIG-I Inflammasome Suppresses Antiviral Immunity and Promotes Inflammation in Asthma and COVID-19.” Nature Communications 14 (1): 2329. https://doi.org/10.1038/s41467-023-37470-4. https://www.nature.com/articles/s41467-023-37470-4.