To date, the coronavirus disease 2019 (COVID-19) pandemic has claimed more than 5.17 million lives worldwide. This pandemic has been caused by the sudden and rapid outbreak of a novel coronavirus, namely, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).
Despite advanced COVID-19 detection tools and vaccine availability, COVID-19 was not contained. This is primarily due to the evolution of the SARS-CoV-2 virus.
A deeper understanding of the pathogenesis and molecular mechanisms of viral infection is needed to develop effective therapeutics.
 Study: Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. Image Credit: 10 FACE / Shutterstock
Study: Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding. Image Credit: 10 FACE / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Role of ACE2 in COVID-19 Infection
Previous studies have revealed that angiotensin-converting enzyme 2 (ACE2) is the primary receptor present on the host cell's surface by which SARS-CoV-2 invades the host cell. ACE2's variability and inducibility determine viral tropism, infectivity, disease progression, or severity.
ACE2 is an important member of the renin-angiotensin system (RAS), and a monocarboxylic peptidase and type I transmembrane protein. In addition, ACE2 contains a large ectodomain, where both viruses (SARS-CoV-1 and -2) bind as well as maintain enzymatically active domains separately.
Scientists indicated that the ectodomain of ACE2 undergoes shedding, which is induced via various stimuli (e.g., bacteria and viruses) and remains active. As a promising COVID-19 therapeutic, scientists have proposed that the soluble ACE2 could be used as a decoy receptor to trap the SARS-CoV-2 virus.
At present, this study is undergoing clinical trials for validation. Another group of researchers debated this strategy because soluble ACE2 could initiate viral entry via an alternative route.
Prior studies have revealed that ACE2 shedding depends upon the activity of a disintegrin and metalloprotease domain 17 (ADAM17), or tumor necrosis factor-alpha converting enzyme (TACE).
ADAM17 genes encode functional proteases and are involved in ectodomain shedding of an array of growth factors, cytokines, and adhesion molecules, including TNF-a and ACE-2.
Previous studies have revealed that the enzymatic activity of ADAM17 could be controlled via posttranscriptional tissue inhibitors of metalloprotease (TIMPs). The expression of Adam 17 is affected by a variety of stimuli.
ACE2 and Placenta
Generally, ACE-2 is found in the outer trophoblast epithelial cell layers of the villous placenta, i.e., at the main functional interface between the mother and the fetus.
During early pregnancy, placental expression levels of ACE-2 are a prominent phenomenon, which progressively decreases throughout gestation.
Some studies have indicated ACE-2 expression occurs only in the trophoblast epithelial cells and not among other stromal cells and fetal endothelium.
ADAM17 has been found to be present in the villous placental trophoblast cells, and its expression has been associated with the production of TNF in inflammatory pregnancy states.
The majority of studies available have focused on evaluating ACE-2 expression in pregnant women infected with SARS-CoV-2 in the third trimester.
Scientists revealed that many women had experienced COVID-19 disease throughout their pregnancies, with a steady low SARS-CoV-2 fetal transmission.
To date, not much evidence has been documented related to ACE-2 expression in various gestational stages of women infected with COVID-19.
A new study, published on the bioRxiv* preprint server, hypothesized that ACE-2 expression and ADAM17 activity in the placenta is affected by the timing of SARS-CoV-2 infection of the mother.
The current study determined ACE-2 expression/ADAM17 activity in placental villous tissues and ACE-2 levels in maternal serum using samples related to the 2nd and 3rd trimester of pregnant women who were infected by SARS-CoV-2.
These results were compared with a control group of healthy pregnant women in a similar time frame. This study design helped understand the trajectory of responses to SARS-CoV-2 infection at the maternal-fetal interface.
The authors documented new evidence on the placental ACE-2 expression in SARS-CoV2 infections in pregnancy. They reported that the changes in the expression in the ACE-2 protein are associated with placental ACE2 shedding, which is mediated by ADAM17 as a result of maternal SARS-CoV-2 infection.
The 3rd-trimester COVID-specific increase in ACE2 mRNA was observed, which might be due to upregulation of ACE-2 transcripts triggered via the active shedding process in this disease state.
This study also revealed that no significant differences in the maternal serum estradiol between our patient groups were found.
This result indicated that other hormonal signaling influenced the expression of placental ACE-2 in these pregnancies. In the future, more research on the evaluation of other factors that influence ACE-2 post-translational regulation and ADAM17 activity is required, especially to elucidate the mechanisms governing this process more clearly.
Researchers indicated the absence of ACE-2 on the fetal endothelium in COVID-19 placental tissues, compared to the lung epithelial cells. This suggests the possibility of ACE-2 as a gatekeeper that prevents perinatal transmission of SARS-CoV-2.
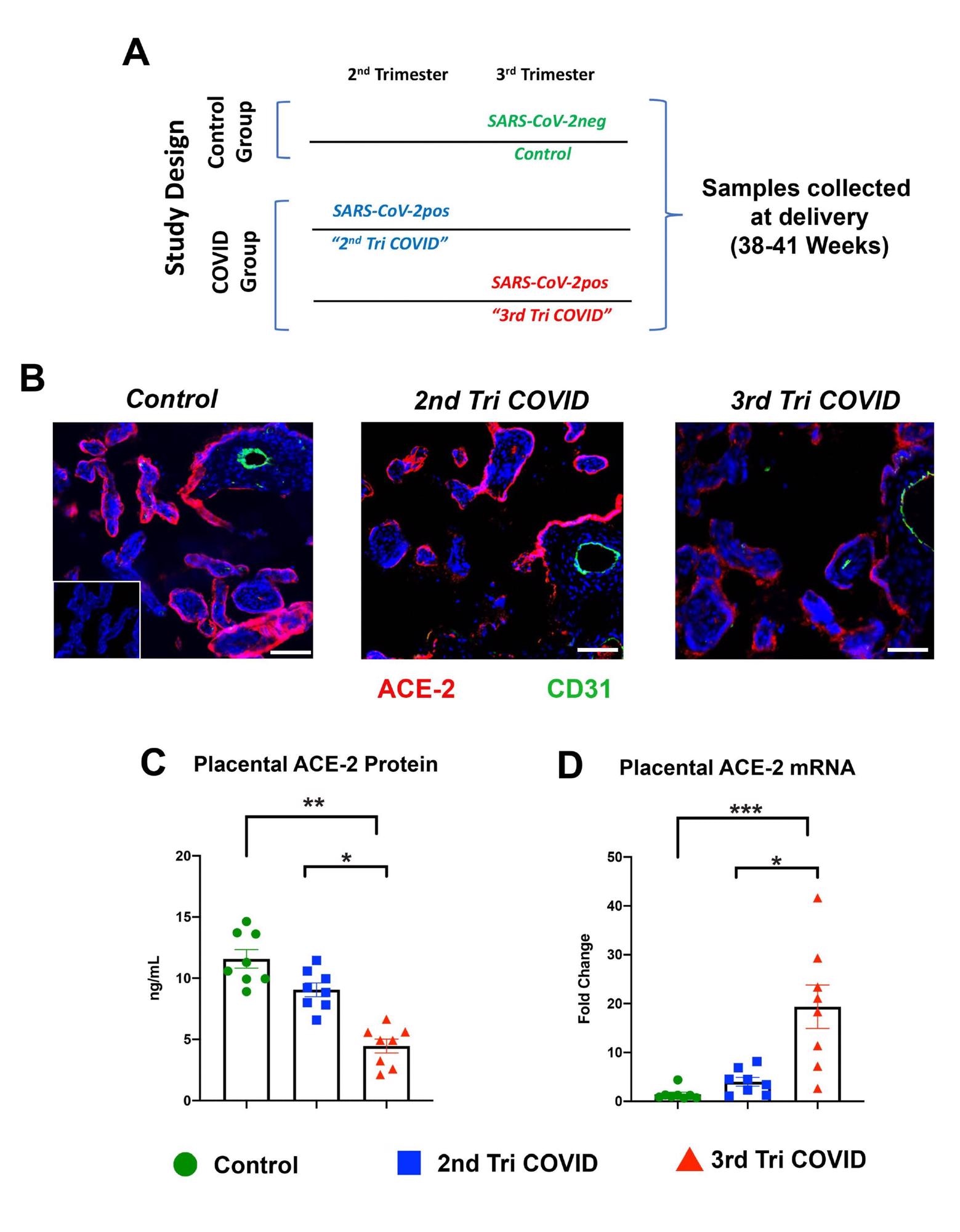 Villous placental ACE-2 expression in acute vs remote SARS-CoV-2 infections in pregnancy. A. Study Design. Control group: pregnancies who had no report of SARS-CoV-2 infection or COVID-19 symptoms during their pregnancy and were SARS-CoV-2 negative via universal screening at time of admission to labor and delivery. COVID group: women with documented COVID-19 symptoms and a SARS-CoV-2 positive test during their 2nd trimester (2nd Tri COVID) or 3rd trimester (3rd Tri COVID) of pregnancy. Schematic shows timing of maternal SARS-CoV-2 infection relative to sample collection at delivery. B. Representative images from immunohistochemical survey of ACE-2 in villous placental tissues from each patient group (n=8 per group). Red: ACE-2, Green: CD31, Blue: DAPI nuclear stain. Inset image: 2nd only antibody negative control. Scale bars: 25mm. VP: Villous placenta; BV: fetal blood vessel. C. ACE-2 expression in villous placental tissue homogenates as assayed by Human ACE-2 ELISA. D qRT-PCR analysis of ACE-2 mRNA expression in villous placental tissues. Error bars: +/- standard error of the mean. * p < 0.05, ** p < 0.01 *** p < 0.001
Villous placental ACE-2 expression in acute vs remote SARS-CoV-2 infections in pregnancy. A. Study Design. Control group: pregnancies who had no report of SARS-CoV-2 infection or COVID-19 symptoms during their pregnancy and were SARS-CoV-2 negative via universal screening at time of admission to labor and delivery. COVID group: women with documented COVID-19 symptoms and a SARS-CoV-2 positive test during their 2nd trimester (2nd Tri COVID) or 3rd trimester (3rd Tri COVID) of pregnancy. Schematic shows timing of maternal SARS-CoV-2 infection relative to sample collection at delivery. B. Representative images from immunohistochemical survey of ACE-2 in villous placental tissues from each patient group (n=8 per group). Red: ACE-2, Green: CD31, Blue: DAPI nuclear stain. Inset image: 2nd only antibody negative control. Scale bars: 25mm. VP: Villous placenta; BV: fetal blood vessel. C. ACE-2 expression in villous placental tissue homogenates as assayed by Human ACE-2 ELISA. D qRT-PCR analysis of ACE-2 mRNA expression in villous placental tissues. Error bars: +/- standard error of the mean. * p < 0.05, ** p < 0.01 *** p < 0.001
Conclusion
Some of the limitations of this study include the small sample size and the gestational evaluation only covering infections in the 2nd and 3rd trimesters of pregnancy.
The authors claimed that this study is the first to document the levels of ACE-2 in serum of pregnant women affected by COVID-19. They reported that ACE-2 levels are increased in pregnant women compared to non-pregnant women.
In the future, more mechanistic studies, including animal models, are required to thoroughly evaluate ACE-2 expression associated with maternal COVID-19 infection in all trimesters of pregnancy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Taglauer, S. E. et al. (2021) Acute SARS-CoV-2 infection in pregnancy is associated with placental ACE-2 shedding, bioRxiv, 2021.11.19.469335; doi: https://doi.org/10.1101/2021.11.19.469335, https://www.biorxiv.org/content/10.1101/2021.11.19.469335v1
- Peer reviewed and published scientific report.
Taglauer, Elizabeth S., Elisha M. Wachman, Lillian Juttukonda, Timothy Klouda, Jiwon Kim, Qiong Wang, Asuka Ishiyama, David J. Hackam, Ke Yuan, and Hongpeng Jia. 2022. “Acute Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Pregnancy Is Associated with Placental Angiotensin-Converting Enzyme 2 Shedding.” The American Journal of Pathology 192 (4): 595–603. https://doi.org/10.1016/j.ajpath.2021.12.011. https://www.sciencedirect.com/science/article/pii/S0002944022000116.