The unprecedented coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has remained a threat to global health since it was first announced on March 11, 2020. Since then, the United States has rolled out two-dose regimens of the BNT162b2/Pfizer and mRNA-1273/Moderna messenger ribonucleic acid (mRNA) vaccines against SARS-CoV-2 in December 2020. Both of these vaccines offer protection against COVID-19-related hospitalization and death for at least six months.
 Study: Antibody titers before and after booster doses of SARS-CoV-2 mRNA vaccines in healthy adults. Image Credit: Tobias Arhelger / Shutterstock.com
Study: Antibody titers before and after booster doses of SARS-CoV-2 mRNA vaccines in healthy adults. Image Credit: Tobias Arhelger / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The transmission of the SARS-CoV-2 B.1.617.2 (Delta) variant increased due to the weakening immunity, thus resulting in a higher number of COVID-19 cases in the summer of 2021. The sudden increase in infections prompted the U.S. regulatory approval of booster vaccines for higher-risk individuals.
Since then, serological studies have revealed that there is a substantial increase in the antibody response from the first to the second dose of the mRNA vaccines. However, the duration and level of antibody response to booster doses after six months of full vaccination are unknown.
Understanding antibody responses to COVID-19 boosters
In a recent study published on the medRxiv* preprint server, researchers measured anti-receptor-binding domain (RBD) immunoglobulin G (IgG) and surrogate virus neutralization of the interaction between the SARS-CoV-2 spike protein and the human angiotensin-converting enzyme (ACE2) receptor, before and after vaccination with boosters in 33 healthy adults. The participants were asked to provide an e-consent form and complete online surveys about their COVID-19 history and vaccination status.
The participants provided finger stick dried blood spot samples before booster administration and 6-10 days after being vaccinated with the booster dose. The results obtained from the study were compared with data collected from a prior community-based study using the same protocols.
The previous community-based study measured the antibody responses after SARS-CoV-2 infection or after receiving the second dose of the mRNA vaccine. The participants were categorized as seropositive or seronegative based on the presence of anti-RBD IgG before vaccination.
Study findings
The data obtained from the study showed that the antibody responses after 6-10 days of receiving the booster dose are greater than natural infection with SARS-CoV-2, after two doses of the mRNA vaccine, and after both natural infection and vaccination. Notably, post-booster IgG levels were higher in females as compared to males and were negatively related to age.
Further, the SARS-CoV-2 Delta variant showed high surrogate neutralization; however, this neutralization response was still lower than that following exposure to the wild-type SARS-CoV-2 strain. No differences were observed for SARS-CoV-2 Delta variant neutralization in females and males; however, the inhibitory concentration of 50% (IC50) was negatively associated with age.
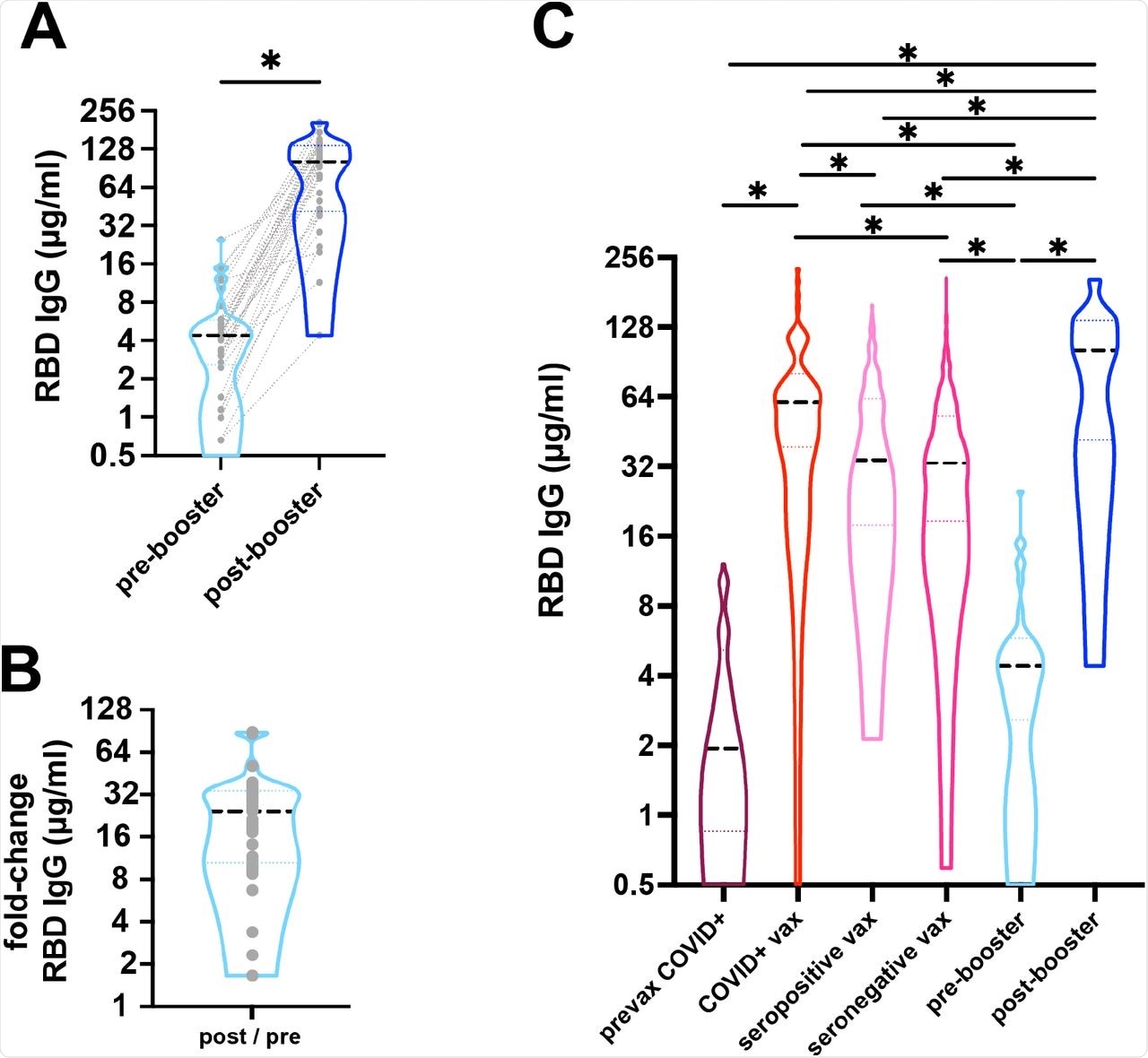 A) Response to COVID-19 mRNA vaccine and booster was measured as anti-RBD IgG antibodies from dried blood spots. Median IgG concentration (black dashed line) increased from 4.4µg/ml pre-booster to 101.6µg/ml post-booster (*p<0.001). Grey dotted lines represents paired samples. n=33. B) There was a median 25-fold change post-booster. C) Median anti-RBD IgG concentration (black dashed line) are shown. Individuals with outpatient COVID-19 had a median of 1.92 µg/ml (n=76) 14-42 days after infection, while individuals with a history of COVID-19 followed by vaccination were higher (60.61µg/ml, n=73, 5-42 days after 2nd dose). Individuals without a known history of COVID-19 who were either seropositive or seronegative and then 2-dose vaccinated had median IgG of 34.15µg/ml (n=181) and 33.09µg/ml (n=687), respectively. Pre-booster levels mean 237.9 days after 2-doses of vaccine were 4.4 µg/ml (n=33) compared to post-booster vaccination level of 101.6 µg/ml (n=33). Dotted lines represent the 25th and 75th percentiles. (*p<0.001).
A) Response to COVID-19 mRNA vaccine and booster was measured as anti-RBD IgG antibodies from dried blood spots. Median IgG concentration (black dashed line) increased from 4.4µg/ml pre-booster to 101.6µg/ml post-booster (*p<0.001). Grey dotted lines represents paired samples. n=33. B) There was a median 25-fold change post-booster. C) Median anti-RBD IgG concentration (black dashed line) are shown. Individuals with outpatient COVID-19 had a median of 1.92 µg/ml (n=76) 14-42 days after infection, while individuals with a history of COVID-19 followed by vaccination were higher (60.61µg/ml, n=73, 5-42 days after 2nd dose). Individuals without a known history of COVID-19 who were either seropositive or seronegative and then 2-dose vaccinated had median IgG of 34.15µg/ml (n=181) and 33.09µg/ml (n=687), respectively. Pre-booster levels mean 237.9 days after 2-doses of vaccine were 4.4 µg/ml (n=33) compared to post-booster vaccination level of 101.6 µg/ml (n=33). Dotted lines represent the 25th and 75th percentiles. (*p<0.001).
Conclusions
The results indicated that the administration of the BNT162b2/Pfizer or mRNA-1273/Moderna boosters may prevent the advancement of infections due to the generation of large antibody responses in healthy adults. Further, the antibody-mediated immunity may be sustained for a longer duration as compared to that which is produced after the second vaccine dose.
Certain limitations associated with the study include limited timeframe, small sample size, and absence of cellular immunity measures. Future studies can further explore the effects of boosters on cell-mediated immunity.
“These data support the use of boosters to prevent breakthrough infections and suggest that antibody-mediated immunity may last longer than after the second vaccine dose.”

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.