Scientists have developed coronavirus disease 2019 (COVID-19) vaccines at unprecedented speed to contain the ongoing pandemic, which has been caused by the rapid spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In addition, a handful of the vaccines have received emergency use authorization from global regulatory bodies.
Currently, about 58% of the total US population is fully vaccinated against COVID-19, and around 69% are partially vaccinated, having received one dose.
Previous studies have shown that SARS-CoV-2 antibodies produced after natural infection can protect an individual against SARS-CoV-2 re-infection. Similar results have been reported by studies using non-human primate models.
In addition, a randomized clinical study associated with subcutaneous administration of REGEN-COV, i.e., a combination of two SARS-CoV-2 neutralizing monoclonal antibodies, or placebo, within 96 hours of exposure to SARS-CoV-2, revealed that REGEN-COV prevented both symptomatic and asymptomatic infection.
A prior longitudinal study, which comprised more than 12,000 health care workers (HCW), showed that SARS-CoV-2 infection-induced immune responses persisted for at least six months.
To determine the efficacy of COVID-19 vaccines, the World Health Organization has recently established the International Standard for Anti-SARS-CoV-2 Immunoglobulin for quantitative evaluation of neutralizing and binding antibody concentrations. They reported that most of the available vaccines provide immune protection two weeks after the second dose of the vaccine.
Although previous studies have quantified the levels of neutralizing and binding antibodies and correlated with protection from symptomatic infection, they failed to compare the results with the antibodies produced after natural infection.
Scientists have recently compared the levels of antibody concentrations in unvaccinated, SARS-CoV-2 convalescent individuals and COVID-19 specific antibodies in vaccinated individuals.
The main focus of this new study is to gain a better understanding of the distribution of antibody concentrations in response to infection and vaccination in a population. This study is available on the medRxiv* preprint server.
About the Study
In this study, the concentration of neutralizing and binding antibodies was estimated using standardized SARS-CoV-2 assays on 3,067 serum samples collected during the period between July 27, 2020, and August 27, 2020, from pre-vaccinated persons with detectable anti-SARS-CoV-2 antibodies using qualitative antibody assays.
The study results were statistically significant, and a strong positive correlation between standardized anti-SARS-CoV-2 RBD IgG and SARS-CoV-2 NT50 concentrations was found irrespective of sex or age categories. This finding is in line with previous studies associated with the effectiveness of the COVID-19 vaccines against SARS-CoV-2 infection. Compared to the time and resource-intensive neutralization assays, researchers have encouraged the usage of high-throughput, commercially available quantitative IgG assays, which are relatively easy when a large-scale cohort is studied.
![SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations and anti-SARS-CoV-2 receptor binding domain (RBD) IgG concentrations for the overall convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay, and according to sex and age category. A) SARS-CoV-2 NT50 concentrations in international units per mL (IU/mL) and B) Anti-SARS-CoV-2 RBD IgG concentrations in binding antibody units per mL (BAU/mL). Horizontal bars represent geometric means, vertical error bars represent 95% confidence intervals, and dashed horizontal lines represent assay cut-off values for seropositivity. P-values from t-tests (sex) and post-hoc Tukey tests (age class) are shown for each sex and age class comparison. Bolded p-values denote statistical significance (p<0.05). Dotted horizontal lines represent antibody concentrations associated with 70% and 90% ChAdOx1 [11] and mRNA-1273 [12] vaccine efficacy (VE). The percentage of sera with antibody concentrations that meet or exceed the concentrations represented by each of the horizontal dotted lines are shown below the charts. SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations and anti-SARS-CoV-2 receptor binding domain (RBD) IgG concentrations for the overall convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay, and according to sex and age category. A) SARS-CoV-2 NT50 concentrations in international units per mL (IU/mL) and B) Anti-SARS-CoV-2 RBD IgG concentrations in binding antibody units per mL (BAU/mL). Horizontal bars represent geometric means, vertical error bars represent 95% confidence intervals, and dashed horizontal lines represent assay cut-off values for seropositivity. P-values from t-tests (sex) and post-hoc Tukey tests (age class) are shown for each sex and age class comparison. Bolded p-values denote statistical significance (p<0.05). Dotted horizontal lines represent antibody concentrations associated with 70% and 90% ChAdOx1 [11] and mRNA-1273 [12] vaccine efficacy (VE). The percentage of sera with antibody concentrations that meet or exceed the concentrations represented by each of the horizontal dotted lines are shown below the charts.](https://www.news-medical.net/images/news/ImageForNews_698126_16383263267937989.jpg) SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations and anti-SARS-CoV-2 receptor-binding domain (RBD) IgG concentrations for the overall convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay, and according to sex and age category. A) SARS-CoV-2 NT50 concentrations in international units per mL (IU/mL) and B) Anti-SARS-CoV-2 RBD IgG concentrations in binding antibody units per mL (BAU/mL). Horizontal bars represent geometric means, vertical error bars represent 95% confidence intervals, and dashed horizontal lines represent assay cut-off values for seropositivity. P-values from t-tests (sex) and post-hoc Tukey tests (age class) are shown for each sex and age class comparison. Bolded p-values denote statistical significance (p<0.05). Dotted horizontal lines represent antibody concentrations associated with 70% and 90% ChAdOx1 [11] and mRNA-1273 [12] vaccine efficacy (VE). The percentage of sera with antibody concentrations that meet or exceed the concentrations represented by each of the horizontal dotted lines are shown below the charts.
SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations and anti-SARS-CoV-2 receptor-binding domain (RBD) IgG concentrations for the overall convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay, and according to sex and age category. A) SARS-CoV-2 NT50 concentrations in international units per mL (IU/mL) and B) Anti-SARS-CoV-2 RBD IgG concentrations in binding antibody units per mL (BAU/mL). Horizontal bars represent geometric means, vertical error bars represent 95% confidence intervals, and dashed horizontal lines represent assay cut-off values for seropositivity. P-values from t-tests (sex) and post-hoc Tukey tests (age class) are shown for each sex and age class comparison. Bolded p-values denote statistical significance (p<0.05). Dotted horizontal lines represent antibody concentrations associated with 70% and 90% ChAdOx1 [11] and mRNA-1273 [12] vaccine efficacy (VE). The percentage of sera with antibody concentrations that meet or exceed the concentrations represented by each of the horizontal dotted lines are shown below the charts.
Researchers reported that around 88% of neutralizing and 63-86% of binding antibody concentrations in this study cohort met or exceeded concentrations associated with 70% COVID-19 vaccine efficacy against symptomatic infection from published VE studies.
Further, this study also reported that around 30% of neutralizing and 14% of binding antibody concentrations met or exceeded concentrations linked with 90% COVID-19 vaccine effectiveness.
This study revealed that all individuals with a history of SARS-CoV-2 infection do not produce antibody responses of suitable magnitude to protect them from re-infection.
Scientists believe this might be one of the reasons why the super spreader SARS-CoV-2 Delta became dominant and increased the global number of COVID-19 cases.
The authors estimated that COVID-19 vaccination increased 2.7 to 8.6-fold higher neutralizing antibody concentrations and 1.5 to 24.2-fold higher binding antibody concentrations.
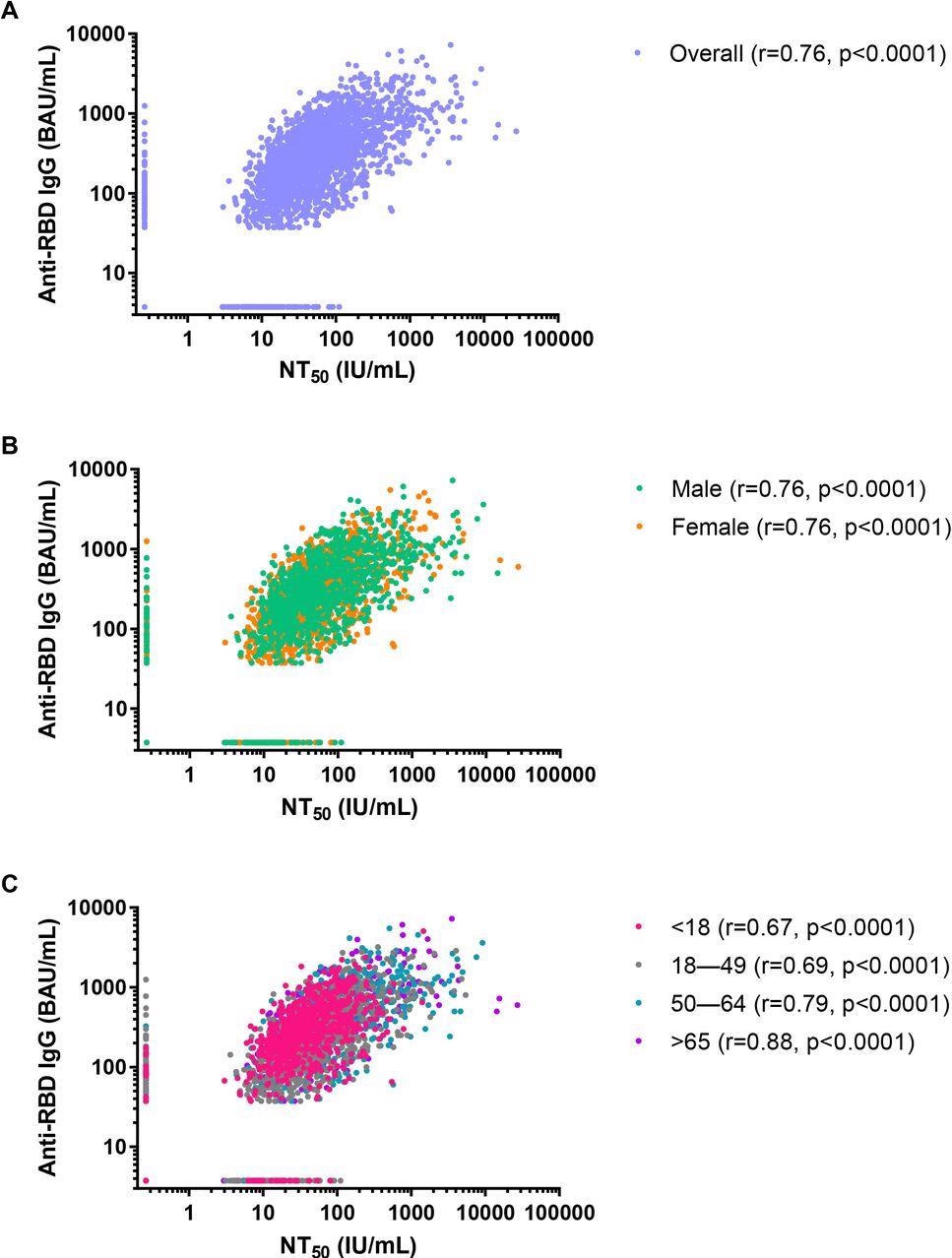 Correlations between SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations in international units per mL (IU/mL) and anti-SARS-CoV-2 receptor-binding domain (RBD) IgG concentrations in binding antibody units per mL (BAU/mL) for the convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay. A) Overall, B) by sex, and C) by age class.
Correlations between SARS-CoV-2 50% neutralizing antibody titer (NT50) concentrations in international units per mL (IU/mL) and anti-SARS-CoV-2 receptor-binding domain (RBD) IgG concentrations in binding antibody units per mL (BAU/mL) for the convenience sample of 3,067 serum specimens collected during July 27,2020-August 27, 2020 with detectable anti-SARS-CoV-2 antibodies on a qualitative assay. A) Overall, B) by sex, and C) by age class.
Limitations of this Study
The current study has several limitations. Firstly, there was a lack of relevant information on the participants, such as if they showed COVID-19 symptoms or date of onset of COVID-19 symptoms and the type of test conducted to determine the levels of antibodies (qRT-PCR or antigen test).
Researchers were unable to confirm the lapse between the onset of infection and the collection of samples for antibody tests. In the clinical trials, the efficacy of COVID-19 vaccines (ChAdOx1 and mRNA-1273) was determined by assessing the levels of antibody concentrations of the serum 28 days after the second vaccine dose. However, while assessing the levels of antibodies after natural infection, samples were likely to be collected within 73 days of SARS-CoV-2 infection. Therefore, there was a high possibility of declining IgG antibody concentrations during the time of sample collection.
At the time of this study, the Delta variant became dominant, which can escape the vaccine-induced immune response. Therefore, a decreased sensitivity to post-vaccination antibody neutralization was observed compared to the Alpha variant of the SARS-CoV-2.
Conclusion
The authors revealed that most non-vaccinated persons revealed qualitative antibody evidence of previous COVID-19 infection whose quantitative estimation of antibodies met or exceeded antibody levels associated with 70% vaccine efficacy.
Only a few participants showed levels of antibody responses that met or exceeded levels associated with 90% of vaccine efficacy.
This finding strongly supported the latest COVID-19 guidance, which stated that all eligible persons must receive COVID-19 vaccination to gain maximum protection against symptomatic COVID-19 irrespective of being previously infected by the virus.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Schuh, J. A. et al. (2021) SARS-CoV-2 Convalescent Sera Binding and Neutralizing Antibody Concentrations Compared with COVID-19 Vaccine Efficacy Estimates Against Symptomatic Infection. medRxiv 2021.11.24.21266812; doi: https://doi.org/10.1101/2021.11.24.21266812, https://www.medrxiv.org/content/10.1101/2021.11.24.21266812v1
- Peer reviewed and published scientific report.
Schuh, Amy J., Panayampalli S. Satheshkumar, Stephanie Dietz, Lara Bull-Otterson, Myrna Charles, Chris Edens, Jefferson M. Jones, et al. 2022. “SARS-CoV-2 Convalescent Sera Binding and Neutralizing Antibody Concentrations Compared with COVID-19 Vaccine Efficacy Estimates against Symptomatic Infection.” Edited by Rafael A. Medina. Microbiology Spectrum 10 (4). https://doi.org/10.1128/spectrum.01247-22. https://journals.asm.org/doi/10.1128/spectrum.01247-22.