Thanks to the relentless efforts of medical researchers around the world, several different messenger ribonucleic acid (mRNA) and vector-based vaccines were authorized for use by regulatory bodies worldwide.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
To date, three vaccines have been authorized for emergency use or FDA-approved in the United States (U.S.) to prevent coronavirus disease 2019 (COVID-19). These vaccines include BNT162b2 (Pfizer-BioNTech - FDA-approved) and mRNA-1273 (Moderna -authorized for emergency use), both of which are mRNA-based vaccines, as well as Ad.26.COV2.S (Janssen -authorized for emergency use).
BNT162b2 and mRNA-1273 are both administered primarily as double-doses, with a third booster shot that has recently been approved after six months. The Ad.26.COV2.S is a replication-incompetent adenoviral vector vaccine and is primarily administered as a single dose; however, the U.S. Food and Drug Administration (FDA) has recently advised Ad.26.COV2.S recipients to receive a booster dose of any approved COVID-19 vaccine after two months.
Guillain-Barré syndrome (GBS) is a rare neurological disorder with an incidence rate of 1-2 per 100,000 person-years. In July 2021, data from the Vaccine Adverse Event Reporting System (VAERS) indicated that more cases of GBS were being reported after vaccination with Ad.26.COV2.S in comparison to other mRNA vaccines.
On July 12, 2021, the U.S. FDA added a warning about GBS to the Ad.26.COV2.S vaccine fact sheet. GBS is being monitored in the Vaccine Safety Datalink as part of ongoing rapid and prospective COVID-19 vaccine safety surveillance efforts.
In a recent study published on the preprint server medRxiv*, researchers compiled available data from the interim analysis of the number of cases of GBS and the risks posed by different vaccines.
Study details
All relevant data for the current study were obtained from the Vaccine Safety Datalink, a collaboration between nine U.S. integrated healthcare systems and the U.S. Centers for Disease Control and Prevention (CDC).
Eight data-contributing organizations contributed data, including Kaiser Permanente in Colorado, Northern California, Northwest, Southern California, and Washington, Marshfield Clinic, HealthPartners, and Denver Health. All organizations had access to comprehensive medical records, including vaccinations for a total of 10,158,003 people over the age of 12 years as of November 10, 2021.
The current study analyzed vaccination data between December 13, 2020, through November 13, 2021. During this time, a total of 14,723,318 doses of COVID-19 vaccines were administered, including 467,126 doses of Ad.26.COV2.S, 8,573,823 doses of BNT162b2, and 5,682,369 doses of mRNA-1273. Eleven cases of GBS after Ad.26.COV2.S were confirmed.
Weekly analyses were used to compare the outcome incidence observed during a risk interval after vaccination between 1-21 days or 1-42 days with outcome incidence expected. The expected outcome incidence was derived from vaccinated comparators who were concurrently (on the same day) in a postvaccination comparison interval between 22-42 days or 43-84 days, respectively. Separate analyses were also obtained from unvaccinated comparators who were concurrently unvaccinated.
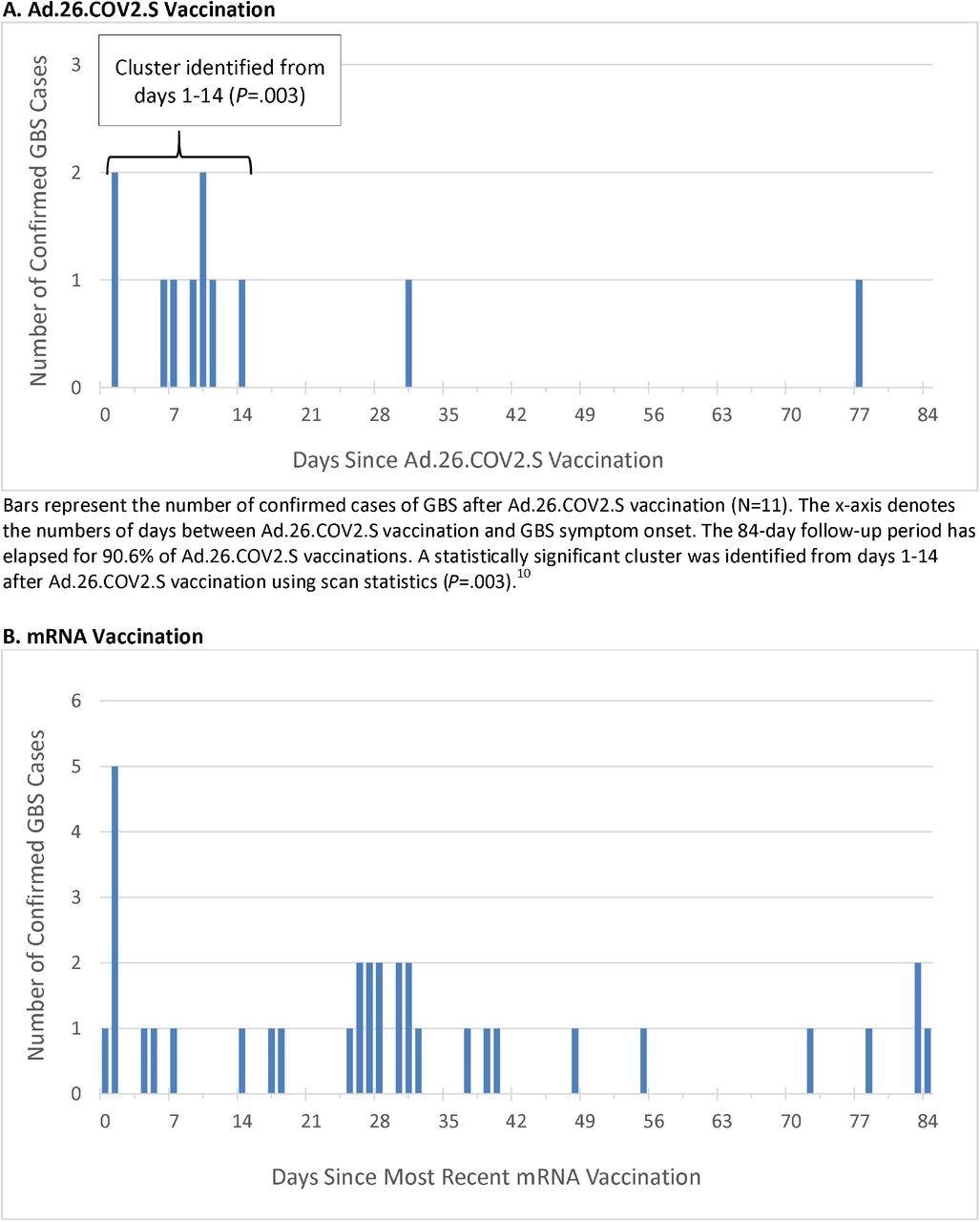 Timing of GBS Symptom Onset after COVID-19 Vaccination
Timing of GBS Symptom Onset after COVID-19 Vaccination
Patients who had received two doses of an mRNA vaccine were considered for analyses 1-21 days after dose 1 and again 1-21 days old after receiving their second dose. However, once they received dose 2, the follow-up time after dose 1 was censored; hence, most of the comparison time in the vaccinated concurrent comparator analyses was after dose 2.
This surveillance approach used two post-vaccination risk intervals, 1-21 days and 1-42 days. The 1-21 day risk interval allowed for on-time analyses and avoided bias introduced from the short interval between two mRNA vaccine doses. However, a 1-42 day risk interval was also used, as this was the standard interval used in vaccine safety studies of GBS and other outcomes.
Potential cases of GBS were identified using International Classification of Diseases 10th Revision (ICD10) code G61.0 in the emergency or inpatient setting, specifically when G61.0 first appeared in an individual’s record in the 1-84 day window after any COVID-19 vaccination.
Since disease onset may begin before a diagnosis is recorded in the medical record, potential cases with the ICD-10 code in the 85-98 day window after vaccination were also reviewed. After review, all cases underwent adjudication according to the Brighton Collaboration criteria.
The unadjusted incidence rate of confirmed cases of GBS per 100,000 person-years in the 1-21 days window post-Ad.26.COV2.S vaccination was 34.6, which is significantly higher than the background rate. The adjusted rate ratios (R.R.) in the 1-21 versus 22-42 day windows following Ad.26.COV2.S vaccination was 6.03.
Thirty-four cases of GBS were confirmed post mRNA vaccination. The unadjusted incidence rate of confirmed cases per 100,000 person-years in the 1-21 day window after administration of mRNA vaccines was 1.4, whereas the adjusted R.R. in the 1-21 versus 22-42 day windows following mRNA vaccination was 0.56. When comparing Ad.26.COV2.S and mRNA vaccinations, the adjusted R.R. was 20.56.
Implications
This interim analysis of surveillance data of COVID-19 vaccines demonstrates an elevated risk of GBS observed after primary Ad.26.COV2.S vaccination. There is ongoing surveillance, and more results are expected soon.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Hanson, K. E., Goddard, K., Lewis, N., et al. (2021). Guillain-Barré Syndrome after COVID-19 Vaccination in the Vaccine Safety Datalink. medRxiv. doi:10.1101/2021.12.03.21266419. https://www.medrxiv.org/content/10.1101/2021.12.03.21266419v1
- Peer reviewed and published scientific report.
Hanson, Kayla E., Kristin Goddard, Ned Lewis, Bruce Fireman, Tanya R. Myers, Nandini Bakshi, Eric Weintraub, et al. 2022. “Incidence of Guillain-Barré Syndrome after COVID-19 Vaccination in the Vaccine Safety Datalink.” JAMA Network Open 5 (4): e228879–79. https://doi.org/10.1001/jamanetworkopen.2022.8879, https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791533