
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The unprecedented surge of SARS-CoV-2 cases has driven a rapid rise in the demand for commercial diagnostic kits. However, such a rapid increase could potentially compromise quality due to the lack of performance and clinical data on these test kits. Therefore, it is crucial to independently evaluate and validate these tests to ensure a minimum acceptable standard before widespread use in the population.
Presently, reverse-transcriptase polymerase chain reaction (RT-PCR) is used widely to detect SARS-CoV-2 from nasal/oral samples; however, the limited access of RT-PCR for symptomatic cases, capacity constraints, and short time window of active infection can underrate the burden of infection. Serological tests, therefore, have become key tools to monitor the incidence of coronavirus disease 2019 (COVID-19).
About the study
In the current study, the researchers evaluated 14 POC antibody tests including 13 lateral flow immunoassays (LFAs) and one microfluidic immunofluorescence assay (Lumira DX). The team tested the specificity and sensitivity of these tests to determine whether they meet the United Kingdom (U.K.) Medicines and Healthcare Products Regulatory Agency (MHRA) standards.
The MHRA established that SARS-CoV-2 LFAs should meet sensitivity and specificity greater than 98% in specimens collected as late as 20 days after the onset of symptoms. The group also analyzed variations in the sensitivity of tests with increasing time of infection and investigated the neutralizing antibody status to establish a correlation with immune status as well as the utility of these tests by patients. Capillary and serum samples were collected in pairs to determine if POC tests fared comparably on capillary samples.
Study findings
The researchers found that only the LumiraDX POC test could match the MHRA standard for both sensitivity and specificity. Other LFAs including those manufactured by Biomerica, Biozek, Fortress, Menarini, and Roche reported over 98% specificity, even for high stringency specificity serum samples.
Surprisingly, the researchers found variations in different batches of Menarini kits reporting 76.3% sensitivity with one batch and 94.9% with another batch for the same set of samples. All LFAs exhibited greater than 90% sensitivity for samples after more than 21 days of symptom onset.
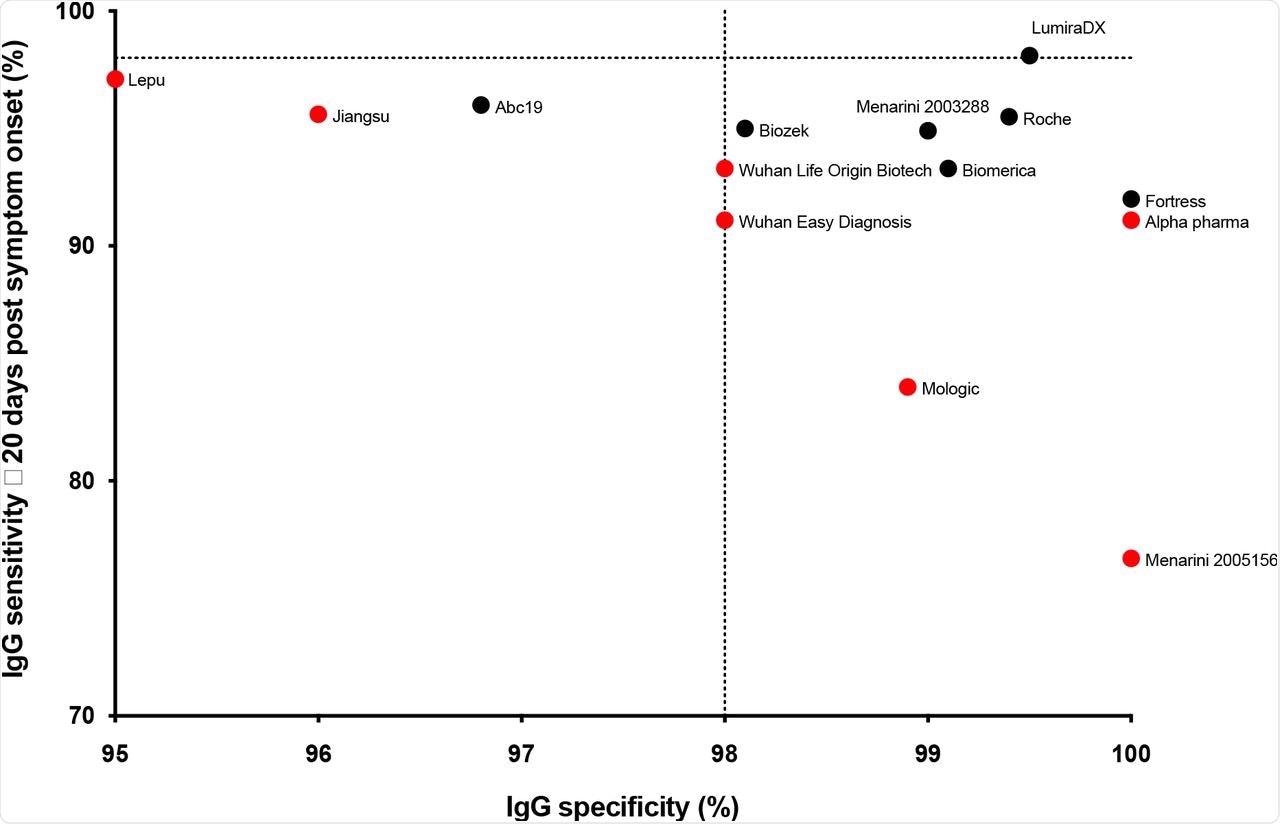
Sensitivity against specificity for the kits tested (IgG only, and positive or negative result for the LumiraDx assay). For each kit, the ≥ 20 days post symptom onset sensitivity is shown. The MHRA targets of 98% sensitivity and specificity are shown as dotted lines. Pharmact was not included on the graph due to low specificity. The LFA that did not progress beyond this stage is shown in red.
The researchers also evaluated the performance of POC kits over time and found that the LumiraDX maintained its consistent sensitivity of over 98% up to 224 days after the onset of symptoms. Neutralizing titers were available for some samples and were established by comparing POC results with half-maximal neutralizing titers (NT50). A significant difference was observed in the NT50 level between positive and negative LFA results and a broad range of NT50 titer values was recorded for positive LFA results.
For paired serum and capillary samples, the serum sample was examined in the lab and interpreted by health care workers (HCW), whereas capillary samples were read by the participant as well as a second HCW. Lower sensitivity was observed in capillary samples as compared to serum samples for five of the seven LFAs.
The magnitude of difference in sensitivity ranged from 19.1 to 34.2% for both Biozek and Roche LFAs, regardless of who read the results. The Fortress LFA reported a slight increase in sensitivity for capillary samples; however, this was not significant statistically. The LumiraDX test showed 100% sensitivity for both samples.
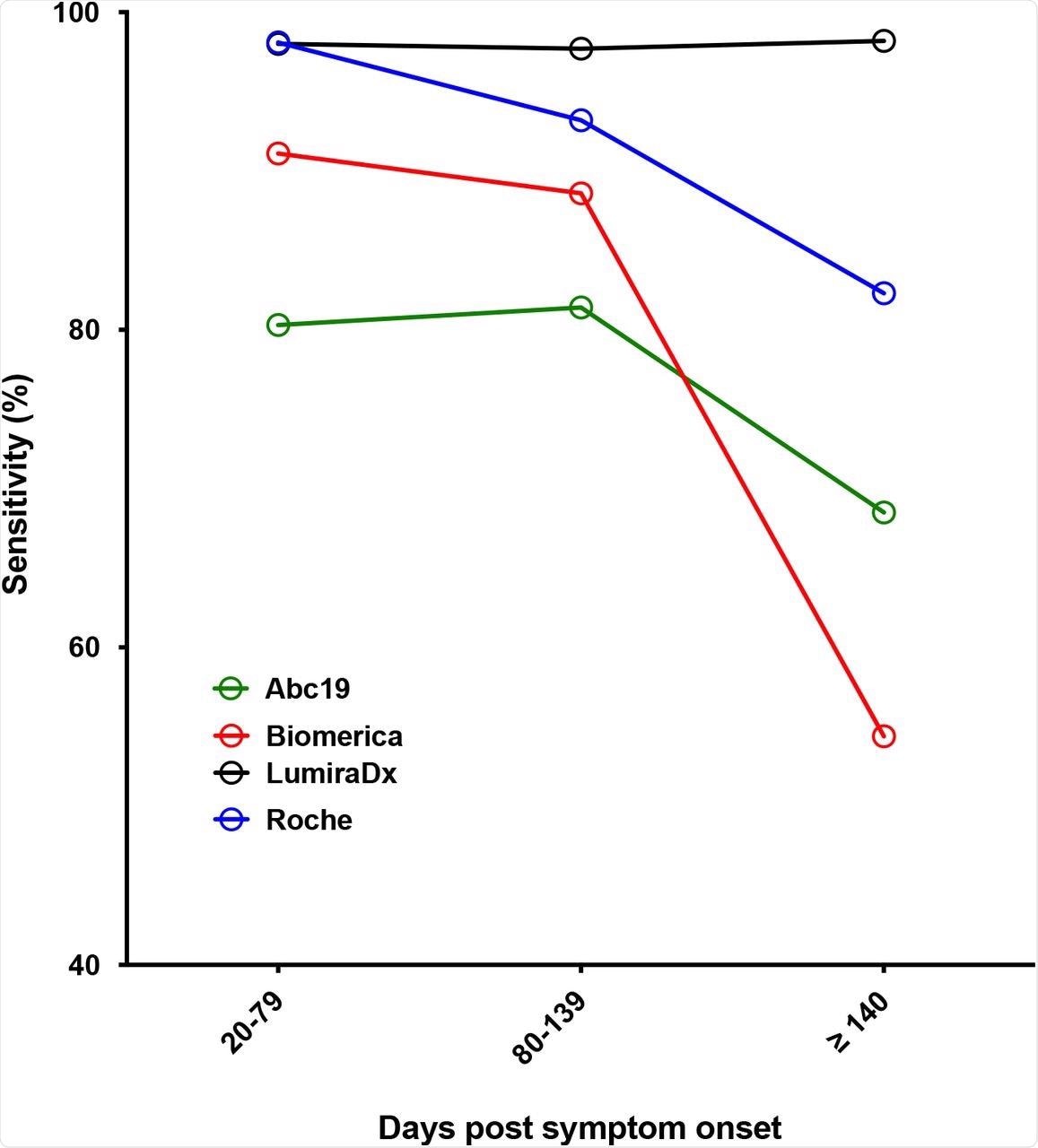
Sensitivity against time post symptom onset for selected kits (IgG for all LFA, with the exception of LumiraDX, where overall antibody result was used).
About 75% of participants claimed that it was very easy or easy to use the kit using the instructions on the leaflet. Furthermore, 89% found it very easy or easy to decipher the explanation of results, whereas 20% reported it is difficult or very difficult to take the sample.
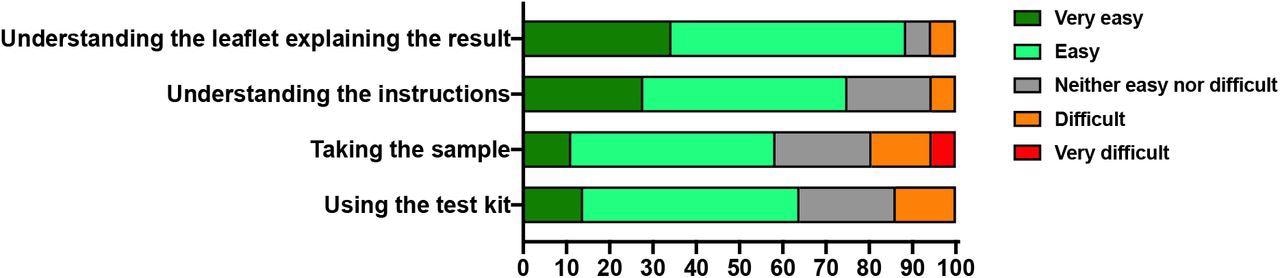 Ease of use Summary of ease of use questionnaire. Participant ease of use responses to the indicated categories was expressed as a percentage of responses.
Ease of use Summary of ease of use questionnaire. Participant ease of use responses to the indicated categories was expressed as a percentage of responses.
Conclusions
The findings of the current study revealed variations between POC kits. Furthermore, several POC tests failed to meet the performance features stated by manufacturers, which underpins the need to establish an independent evaluation of tests. Neutralizing antibody data revealed that these tests could not be used to confer immune status to SARS-CoV-2.
A significant difference was reported in the sensitivity between serum and capillary samples. One of the major advantages of using POC tests is that even ordinary people could perform the test without the need for a skilled HCW.
The observations discussed here highlight the need to optimize POC tests to generate comparable results on both capillary and serum samples. The findings from the current study could help countries identify individuals who may not require imminent vaccination or adjust the vaccine dose to meet the pan-nation vaccination status, given the limited vaccine resources.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
McCance, K., Wise, H., Simpson, J., et al. (2021). Evaluation of SARS-CoV-2 Antibody Point of Care Devices in the Laboratory and Clinical Setting. medRxiv. doi:10.1101/2021.12.16.21267703. https://www.medrxiv.org/content/10.1101/2021.12.16.21267703v1.
- Peer reviewed and published scientific report.
McCance, Kirsty, Helen Wise, Jennifer Simpson, Becky Batchelor, Harriet Hale, Lindsay McDonald, Azul Zorzoli, et al. 2022. “Evaluation of SARS-CoV-2 Antibody Point of Care Devices in the Laboratory and Clinical Setting.” Edited by Gheyath K. Nasrallah. PLOS ONE 17 (3): e0266086. https://doi.org/10.1371/journal.pone.0266086. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0266086.