The lung pathology of severe coronavirus disease 2019 (COVID-19) patients revealed thrombosis as a defining characteristic. Previous studies have shown that clinical indicators of thrombosis, including elevated D-dimer, fibrinogen, and thrombosis-associated inflammatory biomarkers, are present in most COVID-19 patients requiring intensive care.
Study: SARS-CoV-2 Spike protein activates TMEM16F-mediated platelet pro-coagulant activity. Image Credit: Kateryna Kon / Shutterstock.com
About the study
The present study was based on a few observations which suggested that thrombosis in COVID-19 is triggered by local events occurring in the infected lungs. The first observation is regarding the asynchronous deposition of thrombi and fibrin in the lungs. The deposition of these substances appears to be due to relatively recent thrombi infiltrated by inflammatory cells that are close to older thrombi in an advanced stage of fibrotic organization.
The second observation suggests that viral infection triggers local thrombotic events in the lungs due to the sporadic presence of macro- or microvascular thrombosis in other organs. The third observation opposes thrombosis as a result of a systemic consumptive coagulopathy since high D-dimer and fibrinogen levels have been identified in severe COVID-19 patients and no increase in prothrombin time or a decrease in antithrombin levels are observed. Various other observations emphasize the specific involvement of platelets in the pathogenesis of thrombosis in COVID-19 patients.
In the current study, the researchers produced SARS-CoV-2 spike or vascular stomatitis virus (VSV)-G protein-pseudotyped virions, or generated cells expressing the spike protein on their plasma membrane. Subsequently, the researchers examined their effects on platelet adhesion (fluorescence), aggregation (absorbance), exposure of phosphatidylserine (flow cytometry for annexin V binding), calcium flux (flow cytometry for fluo-4AM), and clot formation and retraction.
The researchers discovered a novel mechanism that regulates cell-cell fusion induced by the SARS-CoV-2 spike protein. They began with the observation that the lungs of almost 90% of COVID-19 patients contain several syncytia including two to more than 20 nuclei.
Screening two large libraries of European Medicines Agency (EMA)/ the United States Food and Drug Administration (FDA)-approved small molecules to search for drugs that inhibit spike-induced syncytia formation led to the identification of TMEM16F activity inhibitors niclosamide and clofazimine. Subsequent experiments on the aforementioned cell lines included an evaluation of how niclosamide and clofazimine treatment affects these cells.
“SARS-CoV-2 spike stimulated platelets both when present on the virion envelopes or upon expression onto the plasma membrane of cells.”
Study findings
The study data showed that the exposure of platelets to the SARS-CoV-2 spoke protein increases their activation and adhesion, as well as promotes the release of calcium from these cells. This increase in adhesion and aggregation occurred as a result of the spike protein’s effects on pro-coagulant platelet activation markers, including PS exposure on the platelet outer membrane and generation of thrombin.
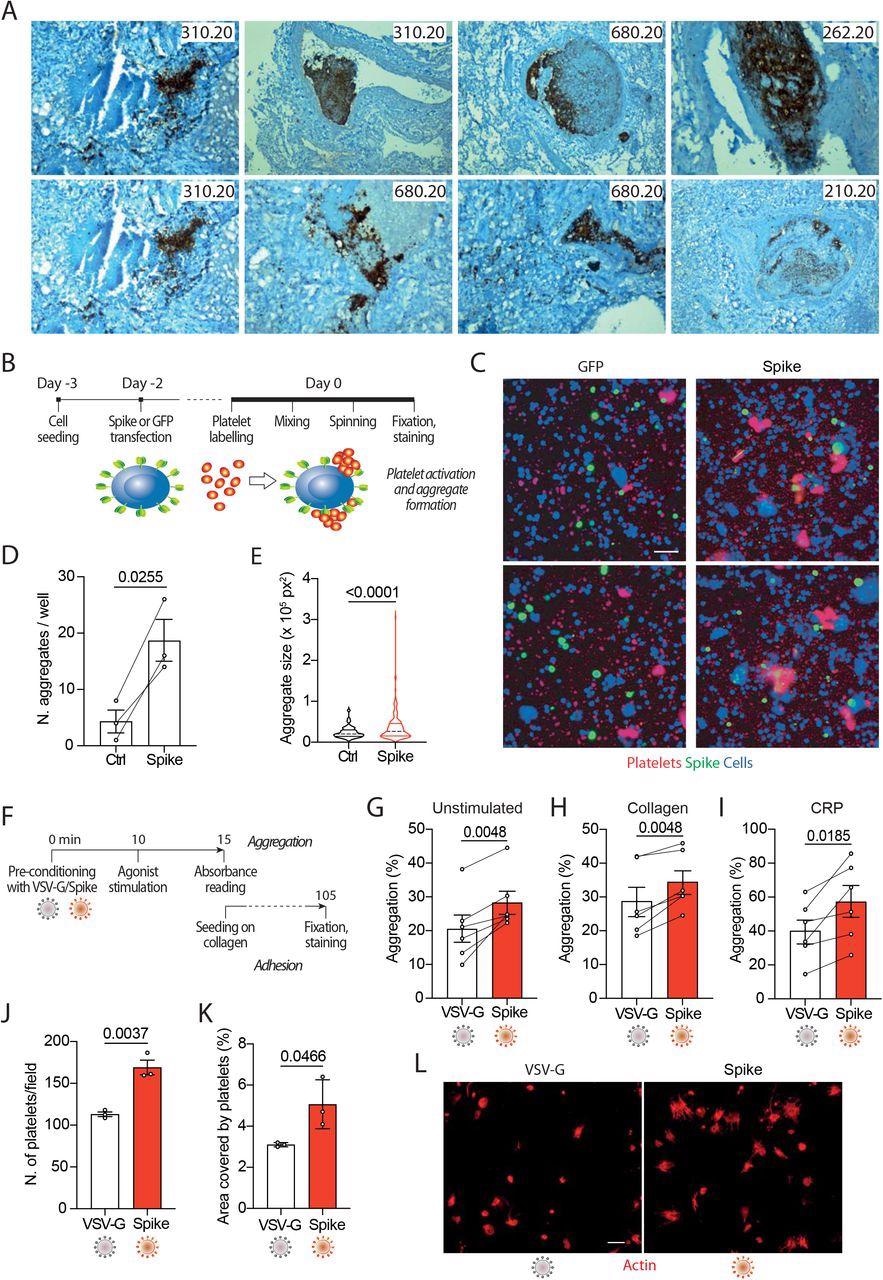 Spike enhances platelet activation. A. Histopathological evidence of platelet aggregates in the thrombotic microvasculature of SARS-COV-2-infected lungs from 4 COVID-19 patients. Numeric codes identify patients. Platelets were stained by using an anti p62 glycoprotein antibody. Magnification: x40 B. Experimental scheme to study platelet activation and aggregate formation. Vero cells transfected to express either Green Fluorescent Protein (GFP) or SARS-CoV-2 Spike were incubated with pre-labeled washed platelets and shaken at 200 rpm for 10 min at 37°C. The plate was centrifuged, fixed, and stained with Cell Mask and antibodies recognizing either GFP or Spike. C. Representative images showing platelet aggregates. Cells stained with Cell Mask are in blue; labeled platelets are in red; GFP or Spike are in green. Scale bar, 20 μm. D. Number of aggregates larger than 40,000 px2. Results are from n=3 independent experiments. Data are mean ± SEM, statistical significance is indicated (paired Student’s t-test). E. Violin plot showing the size of the aggregates found in all the experiments performed. Statistical significance is indicated (unpaired Student’s t-test). F. Experimental scheme for platelet activation in suspension. G-I. Percentage of platelet aggregation when incubated with vehicle (G), stimulated with collagen (H) or CRP (I). Results are from N=6 independent experiments. Data are mean±SEM Statistical significance is indicated (paired Student’s t-test). J. Number of adherent platelets per field. Results are from n=3 independent experiments each performed in duplicate. Each dot represents the mean of 6 images quantified. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test). K. Percentage of the area covered by adherent platelets. Results are from n=3 independent experiments performed in duplicate; each dot represents the mean of 6 images quantified. L. Representative images of platelets adhering on collagen. Images were acquired using a high content fluorescent microscope followed by analysis using the ImageJ software (Fiji). Platelets were stained with F-actin (in red). Scale bar, 5 μm.
Spike enhances platelet activation. A. Histopathological evidence of platelet aggregates in the thrombotic microvasculature of SARS-COV-2-infected lungs from 4 COVID-19 patients. Numeric codes identify patients. Platelets were stained by using an anti p62 glycoprotein antibody. Magnification: x40 B. Experimental scheme to study platelet activation and aggregate formation. Vero cells transfected to express either Green Fluorescent Protein (GFP) or SARS-CoV-2 Spike were incubated with pre-labeled washed platelets and shaken at 200 rpm for 10 min at 37°C. The plate was centrifuged, fixed, and stained with Cell Mask and antibodies recognizing either GFP or Spike. C. Representative images showing platelet aggregates. Cells stained with Cell Mask are in blue; labeled platelets are in red; GFP or Spike are in green. Scale bar, 20 μm. D. Number of aggregates larger than 40,000 px2. Results are from n=3 independent experiments. Data are mean ± SEM, statistical significance is indicated (paired Student’s t-test). E. Violin plot showing the size of the aggregates found in all the experiments performed. Statistical significance is indicated (unpaired Student’s t-test). F. Experimental scheme for platelet activation in suspension. G-I. Percentage of platelet aggregation when incubated with vehicle (G), stimulated with collagen (H) or CRP (I). Results are from N=6 independent experiments. Data are mean±SEM Statistical significance is indicated (paired Student’s t-test). J. Number of adherent platelets per field. Results are from n=3 independent experiments each performed in duplicate. Each dot represents the mean of 6 images quantified. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test). K. Percentage of the area covered by adherent platelets. Results are from n=3 independent experiments performed in duplicate; each dot represents the mean of 6 images quantified. L. Representative images of platelets adhering on collagen. Images were acquired using a high content fluorescent microscope followed by analysis using the ImageJ software (Fiji). Platelets were stained with F-actin (in red). Scale bar, 5 μm.
In severely infected SARS-CoV-2 patients, viral replication is robust in the lung and lower tract respiratory epithelium, which leads to the continuous production of infectious particles. Cells that were engineered to express the SARS-CoV-2 spike protein on their surface were found to fuse with neighboring cells expressing the angiotensin-converting enzyme 2 (ACE2) receptor, which is an essential component of the SARS-CoV-2 entry into host cells. This phenomenon subsequently leads to the formation of large syncytia, which have been observed in over 90% of patients with severe COVID-19.
Taken together, these findings suggest that cells infected with SARS-CoV-2, as well as those that contribute to the formation of large syncytia, contribute to platelet activation and the subsequent induction of thrombosis. Two possible mechanisms that could be responsible for this spike-mediated platelet activation were proposed.
The first potential mechanism suggests that platelet activation could occur directly upon binding of the spike protein to the ACE2 receptor that is expressed in platelets, thereby leading to direct activation of TMEM16F on the platelet plasma membrane. Comparatively, the activation of TMEM16F could be triggered by the increase in calcium that occurs following stimulation with the SARS-CoV-2 spike protein.
In agreement with the first proposed mechanism, the researchers found that the platelets were not activated when the medium was depleted of extracellular calcium, thus indicating that intracellular calcium stores are not required for activation. Platelet activation was also observed occurred upon treatment with isolated spike receptor-binding domain (RBD), which suggests that TMEM16F directly acts on the platelets following the binding of the SARS-CoV-2 spike protein to the ACE2 receptor.
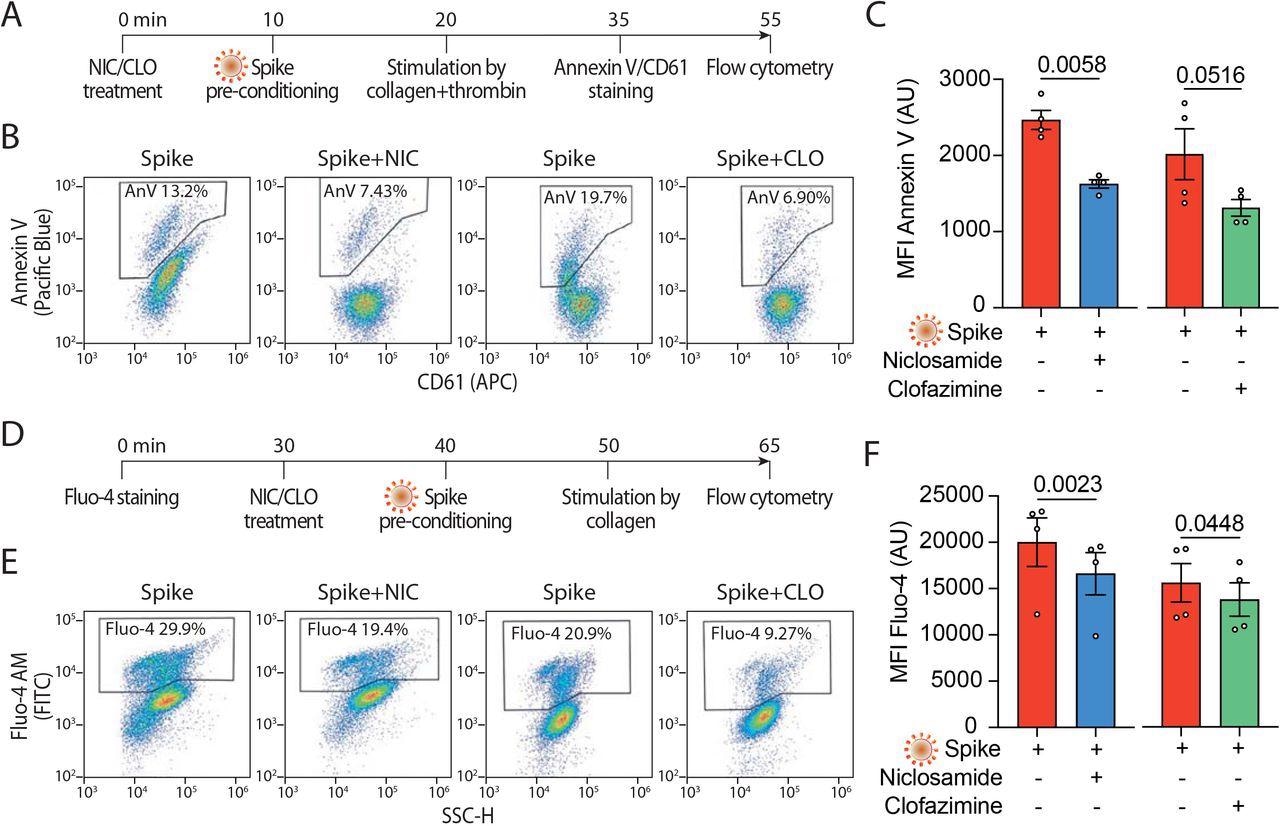 Niclosamide and Clofazimine reduce annexin V and intracellular calcium A. Experimental scheme to assess annexin V reactivity upon Spike stimulation and drug treatment. Platelets were pre-incubated with Niclosamide (NIC, 1 μM) or Clofazamine (CLO, 5 μM) for 10 min, followed by incubation with collagen (30 μg/ml) and thrombin (0.5 units) for 15 min. Platelets were then stained with Annexin V-Pacific Blue and CD61-APC and analyzed by flow cytometry. B. Representative flow cytometry plots. The boxed areas show the percentage of washed platelets positive for annexin V upon pre-treatment with Niclosamide (NIC), Clofazimine (CLO) or vehicle and incubation with VSV-G or Spike pseudo particles, followed by stimulation with collagen and thrombin. C. Mean fluorescence intensity (MFI) of annexin V positive platelets (AU, arbitrary units). Results are from n=4 independent experiments. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test). D. Experimental scheme to assess calcium influx upon Spike stimulation and drug treatment. Platelets were stained with Fluo-4 for 30 min and then pre-incubated with Niclosamide (NIC, 1 μM) or Clofazimine (CLO, 5 μM) for 10 min, followed by incubation with collagen (30 μg/ml) and thrombin (0.5 units) for 15 min. Platelets were then assessed for fluorescence by flow cytometry. E. Flow cytometry plots. The boxed areas show the percentage of washed platelets positive for Fluo-4 upon pre-treatment with Niclosamide (NIC), Clofazimine (CLO), or vehicle (DMSO) and incubation with VSV-G or Spike pseudo particles, followed by stimulation with collagen and thrombin. F. Mean fluorescence intensity (MFI) of Fluo-4 (AU, arbitrary units). Results are from n=4 independent experiments. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test).
Niclosamide and Clofazimine reduce annexin V and intracellular calcium A. Experimental scheme to assess annexin V reactivity upon Spike stimulation and drug treatment. Platelets were pre-incubated with Niclosamide (NIC, 1 μM) or Clofazamine (CLO, 5 μM) for 10 min, followed by incubation with collagen (30 μg/ml) and thrombin (0.5 units) for 15 min. Platelets were then stained with Annexin V-Pacific Blue and CD61-APC and analyzed by flow cytometry. B. Representative flow cytometry plots. The boxed areas show the percentage of washed platelets positive for annexin V upon pre-treatment with Niclosamide (NIC), Clofazimine (CLO) or vehicle and incubation with VSV-G or Spike pseudo particles, followed by stimulation with collagen and thrombin. C. Mean fluorescence intensity (MFI) of annexin V positive platelets (AU, arbitrary units). Results are from n=4 independent experiments. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test). D. Experimental scheme to assess calcium influx upon Spike stimulation and drug treatment. Platelets were stained with Fluo-4 for 30 min and then pre-incubated with Niclosamide (NIC, 1 μM) or Clofazimine (CLO, 5 μM) for 10 min, followed by incubation with collagen (30 μg/ml) and thrombin (0.5 units) for 15 min. Platelets were then assessed for fluorescence by flow cytometry. E. Flow cytometry plots. The boxed areas show the percentage of washed platelets positive for Fluo-4 upon pre-treatment with Niclosamide (NIC), Clofazimine (CLO), or vehicle (DMSO) and incubation with VSV-G or Spike pseudo particles, followed by stimulation with collagen and thrombin. F. Mean fluorescence intensity (MFI) of Fluo-4 (AU, arbitrary units). Results are from n=4 independent experiments. Data are mean±SEM. Statistical significance is indicated (paired Student’s t-test).
Both niclosamide and clofazimine act by blocking spike-induced syncytia formation in a variety of epithelial and non-epithelial cells expressing the ACE2 receptor. The experiments conducted in the current study using these agents found that both niclosamide and clofazimine effectively inhibited spike-induced platelet activation; however, niclosamide was particularly effective at inhibiting platelet activation at low concentrations.
The results of the current study provide evidence for a pathogenic mechanism that might be responsible for COVID-19-induced thrombosis. These findings also support the repurposing of niclosamide, which targets TMEM16F, for the treatment of COVID-19.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.