
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Significance of Omicron in immunocompromised patients
The severe acute respiratory syndrome-associated coronavirus disease (SARS-CoV-2) Omicron variant was declared as a variant of concern (VOC) in November 2021. While Omicron is thought to mostly escape the antibody neutralization elicited by the COVID-19 vaccines, the T cell responses against SARS-CoV-2 Omicron variant post-vaccination might be preserved. Therefore, it is essential to determine the modifications of the immune response against Omicron post third dose of COVID-19 vaccination, especially in immunocompromised patients with weakened antibody responses.
The study
The present monocentric study was conducted from March 2021 at the University Hospital Geneva, Switzerland, to determine Omicron spike (S) protein-associated T cell responses before and after the third dose of mRNA COVID-19 vaccine in anti-CD20-treated patients. The study was conducted as per the Good Clinical Practices (GCP) guidelines.
After submitting an informed consent, a total of 20 adults under anti-CD20 monotherapy with ocrelizumab for MS were recruited for the study. The participants received their third dose of the mRNA COVID-19 vaccine six to seven months after the primary two-dose regimen. Sixteen subjects received the mRNA-1273 vaccine, and four subjects received the BNT162b2 vaccine.
The inclusion criteria were those on ocrelizumab treatment for MS and those who received third dose mRNA COVID-19 vaccination before November 1, 2021. The subjects' serum and peripheral blood mononuclear cells (PBMC) samples were collected on the day of the third dose COVID-19 vaccination and one-month post-third-dose vaccination. In addition, the total antibodies of anti-SARS-CoV-2 receptor binding domains (RBD) and anti-SARS-CoV-2 nucleoprotein (anti-N) were measured.
The CD4 and cytotoxic CD8 T cells specific for the SARS-CoV-2 S protein of Delta and Omicron variants and vaccine strain were quantified by activation marker induced (AIM) assay. Similarly, the frequency of T cells specific to the S protein of the vaccine strain, Delta, and Omicron variants were compared before and after third-dose vaccination.
Results
According to the findings, the median duration between the primary two-dose vaccine regimen and the third booster vaccination was 26.7 weeks. The seropositivity for anti-RBD-antibodies increased from 55% to 65% one month after the third vaccine dose. One of the participants was infected by SARS-CoV-2 before vaccination, which was estimated by the presence of anti-N antibodies.
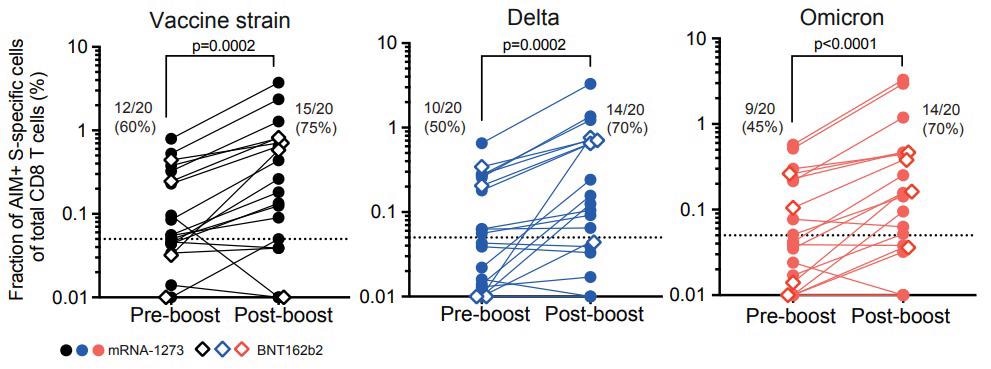
CD8 T-cell responses specific to vaccine strain and variants Delta and Omicron are boosted after the third vaccine dose. Graphs summarize data from n=20 ocrelizumab-treated individual patients with their geometric mean. One dot represents one patient and lines connect pre- and post-boost vaccination frequencies of AIM+ CD8 T cells specific for SARS-CoV-2 vaccine strain (left), the variant Delta (middle), and Omicron (right) and are background subtracted. The dotted line represents the limit of detection. Percentages of responders (those with levels above the limit of detection) are indicated.
Before booster vaccination, the AIM-induced CD8 T cells specific to the SAR-CoV-2 S proteins of the vaccine strain, Delta variant, and Omicron variant were detected in 60%, 50%, and 45% of the population, respectively. After the booster dose, the proportion increased to 75% in vaccine strain, and both Omicron and Delta variants had about 70%. Before booster dose, the median frequency of SAR-CoV-2 S protein-specific CD8 T cell response was 83% in Delta and Omicron variants and about 79% in the vaccine strain. After the third vaccine dose, the frequency of Delta (89.3%)- and Omicron (71%) variant-specific CD8 T cell response was lower compared to the frequency of vaccine strain.
Before the third vaccine dose, the frequency of patients with detectable CD4 T-cell responses in all variants was between 45-50%, and after the booster dose, the frequency increased to around 70-75% in the SARS-CoV-2 vaccine strain and Delta variant, whereas the frequency remained at about 55% in the Omicron variant. The median frequencies for SARS-CoV-2 S protein-specific CD4 T cell response before the third vaccine dose were 72.2% for Delta and 62.5% for the Omicron variant. After the third vaccine dose, median frequencies for the S protein-specific CD4 T cell response were 83.5% for Delta and 72.3% for the Omicron variant.
Conclusions
The results indicated that vaccinated MS patients under ocrelizumab treatment had significant T cell responses towards S protein from Omicron, Delta variants, and vaccine strain. Moreover, the cytotoxic T cell responses increased after the third booster dose.
Although the S protein-specific CD4 and CD8 T cells against all SARS-CoV-2 variants were detectable in around 50% of the study population six months post-second COVID-19 vaccine dose, lower frequencies were associated with Omicron and Delta variants. After the third mRNA vaccine dose, S protein-specific T cell immunity was enhanced, especially the cytotoxic CD8 T cell responses. However, the frequency of T cells specific to Delta and Omicron variants was lower than T cells specific to the vaccine strain both before and after the booster dose. The clinical significance of reduced frequencies of Omicron-specific T cells needs further evaluation in the future.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Omicron-specific cytotoxic T-cell responses are boosted following the third dose of mRNA COVID-19 vaccine in anti-CD20-treated multiple sclerosis patients, Natacha Madelon, Nelli Heikkila, Irene Sabater Royo, Paola Fontannaz, Gautier Breville, Kim Lauper, Rachel Goldstein, Alba Grifoni, Alessandro Sette, Claire-Anne Siegrist, Axel Finckh, Patrice H Lalive, Arnaud M Didierlaurent, Christiane S Eberhardt, medRxiv, 2021, doi: https://doi.org/10.1101/2021.12.20.21268128, https://www.medrxiv.org/content/10.1101/2021.12.20.21268128v1
- Peer reviewed and published scientific report.
Madelon, Natacha, Nelli Heikkilä, Irène Sabater Royo, Paola Fontannaz, Gautier Breville, Kim Lauper, Rachel Goldstein, et al. 2022. “Omicron-Specific Cytotoxic T-Cell Responses after a Third Dose of MRNA COVID-19 Vaccine among Patients with Multiple Sclerosis Treated with Ocrelizumab.” JAMA Neurology 79 (4): 399–404. https://doi.org/10.1001/jamaneurol.2022.0245. https://jamanetwork.com/journals/jamaneurology/fullarticle/2789588.