Severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infects the kidneys and contributes to tissue scarring, as shown by researchers from the RWTH Uniklinik Aachen, Germany, and Radboudumc, The Netherlands. The developed scar tissue in the infected kidneys may suggest a possible impact on kidney outcomes in the long term.
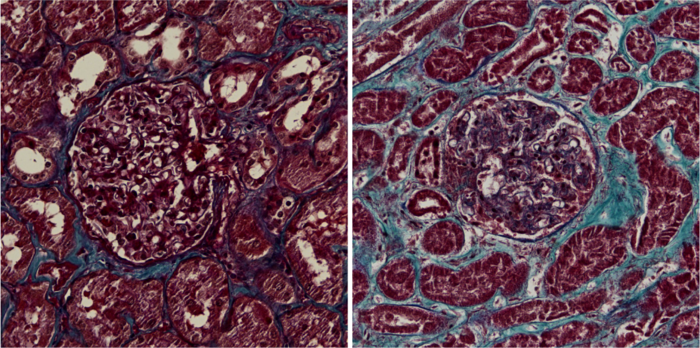
Kidney sections from healthy control (left) and COVID-19 patient (right). Scar tissue is blue. Image Credit: Jitske Jansen and Bart Smeets, Radboudumc
The fact that the Coronavirus can result in severe damage in the human body is known, and also that kidneys can get infected. But what exactly happens in the kidney as a result of the infection, remains elusive until now. In this study, published in Cell Stem Cell, researchers investigated the kidney tissue of COVID-19 patients admitted to the Intensive Care Unit. They found scarring of the tissue as compared to Intensive Care patients with a non-COVID-19 lung infection and a control group.
Next, the researchers questioned what exactly the cause was of the kidney damage. Could this be a direct effect of the virus, independent of systemic inflammation? To investigate this, the researchers cultured mini kidneys in the lab, called organoids. The kidney organoids are developed from stem cells and contain many different kidney cells, except immune cells. The kidney organoids were infected with SARS-CoV-2 and the researchers investigated the direct effect of the virus on the kidney cells, independent of potential secondary effects caused by immune cells or other systemic effects. The researchers found, in line with the COVID-19 patient tissues, scarring of the kidney organoids and accompanied signals that contribute to the scarring process.
The results of this study indicate that the recent finding of another USA-based large cohort study that reported kidney functional decline in over 90,000 COVID-19 survivors (Bowe et al JASN) might be due to direct effects of the SARS-CoV2 virus on the kidney causing scar formation.
Researcher Jitske Jansen (Radboudumc): "In our study, we thoroughly investigated the causal damaging effects of the Coronavirus in the kidneys. The infected kidney organoids show that the virus directly causes cell damage, independent of the immune system. With this work, we found a piece of the puzzle showing the deleterious effects the virus can have in the body". Researcher Katharina Reimer (RWTH Aachen Uniklinik): "Kidney fibrosis, or scarring, is a serious long-term consequence that can occur virtually after any injury to the kidney and correlates with kidney function. Our work shows kidney scarring in COVID-19 patients, which provides an explanation why the virus might cause kidney functional decline as demonstrated in other studies. Long-term follow-up studies will provide further insights into kidney-related pathologies caused by SARS-CoV-2".
Journal reference:
- SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids – Jitske Jansen, Katharina Charlotte Reimer, James Shiniti Nagai, Finny S. Varghese, Gijs J. Overheul, , Ronald P. van Rij, Ivan G Costa, Rebekka K. Schneider, Bart Smeets and Rafael Kramann. DOI: https://doi.org/10.1016/j.stem.2021.12.010