Biological studies have shown that a third booster dose leads to increased antibody levels and gives increased protection against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In Israel, individuals 16 years or older were eligible for vaccination from February 2021, while those 12-15 years old became eligible for vaccination in June 2021. From July 30, 2021, a third booster dose of the BNT162b2 vaccine was also given to residents 60 years or older to restore waning immunity.
Study: Protection following BNT162b2 booster substantially exceeds that of a fresh 2-dose vaccine: a quasi-experimental study. Image Credit: guteksk7 / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the current study, researchers utilized the changes made to the vaccine eligibility age cutoffs in Israel to design a quasi-experimental design. To this end, they compared the protection conferred by two doses of the BNT162b2 vaccine to that given by a third booster dose over the four-week study period.
In the primary analysis, researchers compared the rates of confirmed SARS-CoV-2 infections in two cohorts between September 12, 2021, and October 9, 2021. While the first cohort consisted of recently boosted individuals in the 16-18 age group, the second cohort included individuals in the 12-14 age group who were doubly vaccinated in less than 60 days to avoid the effect of waning immunity. Since there were only a few cases of severe coronavirus disease 2019 (COVID-19) reported from these cohorts, they were excluded from the study.
The secondary analysis compared the rates of confirmed infections across three cohorts. Both the first and third cohorts consisted of individuals in the 16-18 age group. However, those in the first cohort were recently boosted, whereas those in the third cohort were recently vaccinated with two vaccine doses. The second cohort consisted of individuals in the 12-14 age group who had recently taken two vaccine doses.
Study findings
The primary analysis results showed that a booster dose of the BNT162b2 messenger ribonucleic acid (mRNA) vaccine provided a 3.7-fold increase in protection against confirmed infection compared to two doses of the same vaccine. The infection rate in the booster cohort was 3.3 per 100,000 at-risk days, as compared to 12.4 in the doubly vaccinated cohort.
These results complement other lab-based findings showing increased antibody responses both in the immunoglobulin G (IgG) titers and neutralizing antibody levels of the booster dose. Moreover, the neutralizing antibodies after the booster were superior to antibodies elicited after a second dose against both the SARS-CoV-2 Delta and Omicron variants, as indicated in the results of neutralization assays.
Secondary analysis results showed that in the 12-14 years vaccinated cohort, the increase in protection was 2.1-fold lower than in the booster cohort, with a 12.5-fold reduction in infections as compared to the unvaccinated group of that age. In the 16-18 years vaccinated cohort, protection was 2.7-fold lower than the booster group, with the rate of infection 9.8-fold less than the unvaccinated group.
Infection rates in the booster cohort were 26.3-fold lower than in the corresponding unvaccinated group. Overall, this analysis suggests that the response in the 12-14 years vaccinated cohort was similar to the booster group.
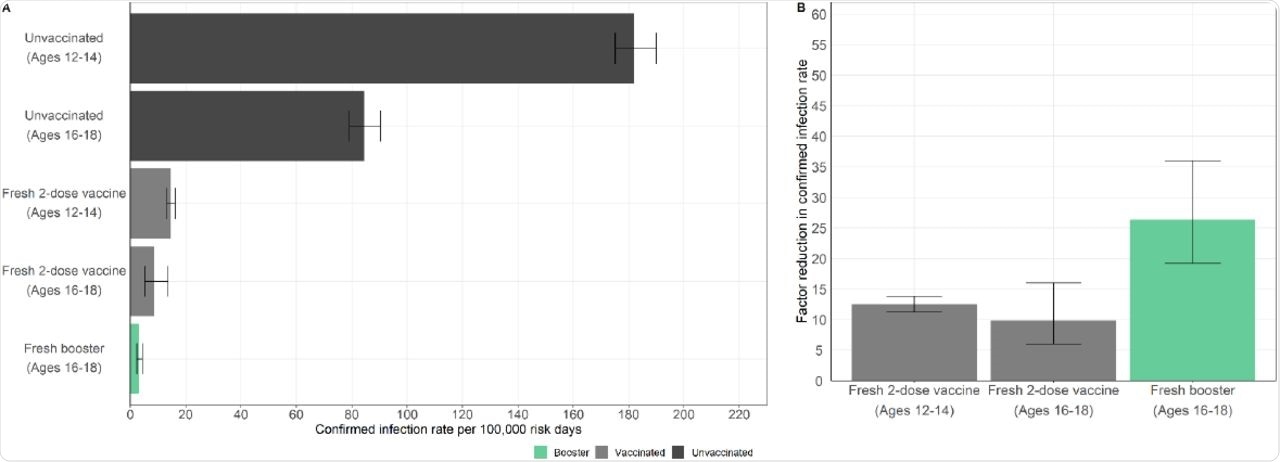
Protection compared to unvaccinated individuals.
Furthermore, the 16-18 years vaccinated cohort showed similar exposure risk to that of the unvaccinated cohort of the same age group. While it did not suffer from age bias, this group showed a behavioral bias, as they were vaccinated relatively late.
The sensitivity analyses results showed low infection rates in the unvaccinated individuals in the 17-18 years age group and a 4.6-fold increase in protection for a booster dose for 16-year-old as compared to doubly vaccinated 14-year-olds. Since booster uptake rates were higher in the Jewish population, an additional analysis of this sub-population was conducted, which showed 4.1-fold improved protection after a booster dose.
The analysis of confirmed infection rates from September 12, 2021, to October 24, 2021, showed a 3.5-fold increase in protection for the booster cohort. Overall, all sensitivity analyses estimated a 3–5-fold increase in protection from a fresh booster dose.
Conclusions
The quasi-experimental nature of the study introduced several biases resulting from the different age groups in the cohorts, which also made a comparison of the effectiveness of the booster dose with that of two doses challenging. The researchers explored the magnitude of these biases, including observed higher confirmed infection rates in the cohort of individuals of 16 years or more and lower rates in individuals of 17 years and above. Additional biases included those due to the sociodemographic status of individuals who received the booster dose early, as they chose to get vaccinated earlier.
To address these biases, the researchers conducted sensitivity analyses and compared the booster cohort with the vaccinated cohorts using the unvaccinated cohort in the corresponding age groups as a baseline reference. Nevertheless, the study rightly estimated the real-world effectiveness of the Pfizer-BioNTech vaccine, including indirect effects such as added protection due to more vaccinated individuals.
Together, the study findings provide a scientific basis for including a third booster dose as part of the BNT162b2 vaccination regimen and suggest that booster doses improve protection beyond two doses of vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Amir, O., Goldberg, Y., Mandel, M., et al. (2021). Protection following BNT162b2 booster substantially exceeds that of a fresh 2-dose vaccine: a quasi-experimental study. medRxiv. doi:10.1101/2021.12.19.21267933. https://www.medrxiv.org/content/10.1101/2021.12.19.21267933v1.
- Peer reviewed and published scientific report.
Amir, Ofra, Yair Goldberg, Micha Mandel, Yinon M. Bar-On, Omri Bodenheimer, Nachman Ash, Sharon Alroy-Preis, Amit Huppert, and Ron Milo. 2022. “Protection Following BNT162b2 Booster in Adolescents Substantially Exceeds that of a Fresh 2-Dose Vaccine.” Nature Communications 13 (1). https://doi.org/10.1038/s41467-022-29578-w. https://www.nature.com/articles/s41467-022-29578-w.