Israel, like most other countries around the world, has experienced several outbreaks of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The third wave of coronavirus disease 2019 (COVID-19) cases in Israel began in September 2020, partly due to the entrance of the Alpha SARS-CoV-2 variant. At the peak of this wave, almost 8,000 new cases were reported every day.
Background
Israel was one of the first countries to authorize the use of the Pfizer-BioNTech messenger ribonucleic acid (mRNA) BNT162b2 vaccine. Soon after, the United States Food and Drug Administration (FDA) also granted emergency approval to the BNT162b2 vaccination after it demonstrated 95% efficacy over a two-month follow-up period.
The period of protection conferred by COVID-19 vaccination is of significant concern. Initially, Pfizer-BioNTech claimed a 91% efficacy rate over a six-month follow-up period and an expected 6% drop in efficacy every two months in their third-phase research data. Given that most people in Israel have been fully vaccinated, population-based observational studies are no longer a viable approach to evaluating the vaccine's long-term effectiveness.
SARS-CoV-2 infection rates in Israel rose again between June and September 2021 as a result of the emergence of the Delta variant (B.1.617.2). Initial serologic research on the Delta variant suggests that the BNT162b2 vaccination protects against infection with the Delta variant, but at a lesser rate than the Alpha variant and 88% versus 93.7%, respectively.
About the study
In a recent study published in the U.S. Centers for Disease Control and Prevention (CDC) Emerging Infectious Diseases journal, researchers were interested in determining whether BNT162b2 vaccination has become less efficient in preventing infection. If so, the researchers sought to identify which population groups and to what extent they were affected by this drop in vaccine efficacy.
To do this, the authors conducted three distinct analyses to address the following questions in order to achieve this goal. First, do neutralizing antibody levels in vaccinated individuals drop over time, and if so, for whom and how quickly? Second, what is the link between antibody levels and polymerase-chain-reaction (PCR)-confirmed SARS-CoV-2 infection? Finally, is there a difference in PCR-confirmed infection rates between those who were immunized early in the immunization program and those who were vaccinated later?
Study findings
The research population's serologic levels fell with time, from a mean of 14,008 for those tested within a month of vaccination to a mean of 1,411 for those tested six months following vaccination. When the results were stratified by age group, sex, socioeconomic level, and chosen chronic conditions, the researchers observed a reduction with time in all demographic groups. The most significant mean differences between subpopulations were found in their baseline serological levels.
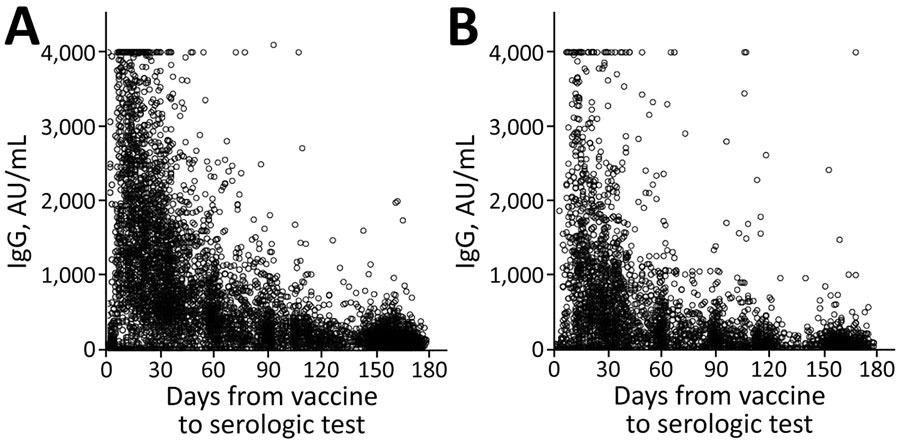
IgG levels for population vaccinated with mRNA BNT162b2 vaccine (Pfizer-BioNTech, https://www.pfizer.com) against coronavirus disease over time, by age group, Maccabi Healthcare Services, Israel, January‒June 2021. A) <60 years of age. B) >60 years of age.
In the first month, mean serologic levels for individuals over the age of 60 were around half of those for participants younger than 60 years old. However, this difference faded to a 10% difference six months later.
Participants with chronic illness, particularly those with an immunosuppressive problem, chronic kidney disease, or cardiac disease, had significant disparities in baseline serologic levels. Initial serologic levels rose as one's socioeconomic status rose.
A positive PCR result was found in 2.8% of people who received both doses of the BNT162b2 vaccine. This group, like others, saw similar drops in serologic means month after month. The mean serologic level among individuals examined in the first 7–30 days, on the other hand, was significantly greater than the overall study population.
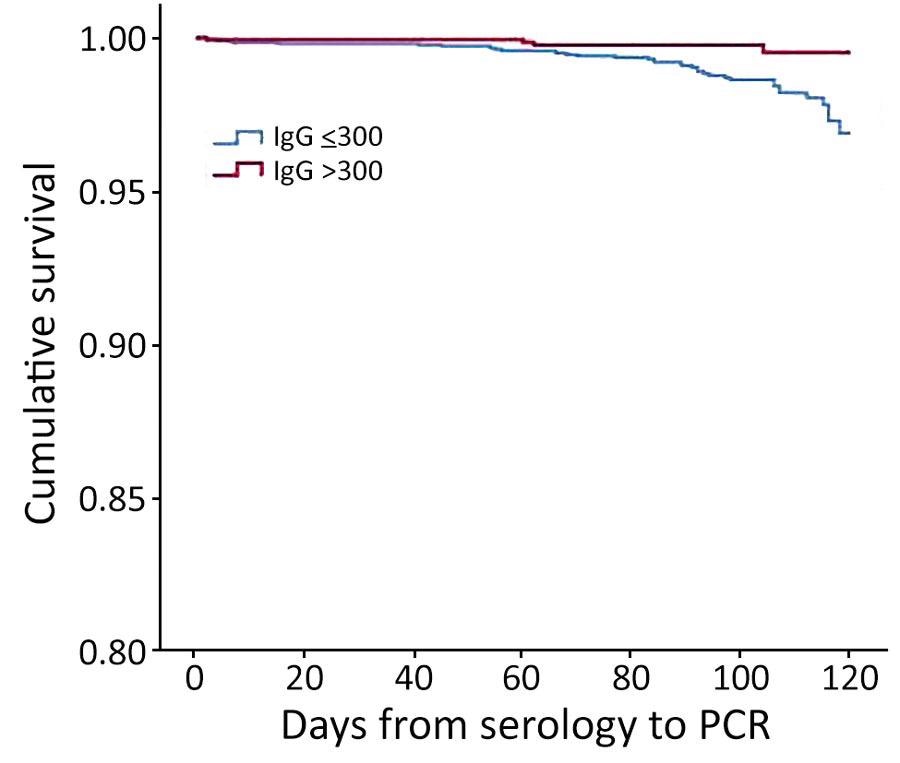
Kaplan-Meier cumulative survival for PCR-positive outcome for population vaccinated with mRNA BNT162b2 vaccine (Pfizer-BioNTech, https://www.pfizer.com) against coronavirus, by antibody (IgG) level, Maccabi Healthcare Services, Israel, June‒July 2021.
Of the 5,141 people who had both serologic and PCR tests, 57% had a serologic test result of 150 AU/mL, 6% had a result of 150-299 AU/mL, 10% had a result of 300–799 AU/mL, and 27% had a result of greater than 800 AU/mL. The proportion of participants with a positive PCR result was 1.2% for those with serologic levels of 150 AU/mL, 1.3% for those with levels of 150–299 AU/mL, 0.2% for those with levels of 300–799 AU/mL, and 27% for those with levels of greater than 800 AU/mL.
The mean serologic level for the 42 research participants who received a positive PCR result was 175 AU/mL as compared to a mean serologic level of 2,057 AU/mL for those who did not receive a positive result. Of the study participants, 365 (7%) had previously been infected, 37% of whom received 1 vaccine dose. A
As a result, those who had previously been infected were less likely to have a serologic level of 300 AU/mL. However, 0.8% of those tested had a PCR positive result, regardless of a previous infection.
Eighty-six percent of those eligible for immunization had received both vaccine doses in the Health Maintenance Organization at the time of the study. The researchers examined the demographic and health characteristics of those who were vaccinated within the first two months to those who were vaccinated later. Those who received their vaccinations during the first two months were more likely to be older, from a higher socioeconomic background, and have a greater risk of chronic illness.
PCR positive results were detected in 1,518 of those vaccinated between January and February 2021, as compared to 644 of those vaccinated between March and May 2021. Age, sex, socioeconomic level, and prevalence of chronic conditions were all linked to a positive PCR result in univariate analysis.
In a logistic regression model, socioeconomic position, age group, vaccination period, sex, and heart disease were all linked to subsequent infection. When all other characteristics were considered, the scientists discovered those study participants who were vaccinated first were 1.6 times more likely to contract COVID-19 than those who were immunized later.
Implications
The authors discovered that serologic levels declined with time for all groups and that there was a link between serologic levels and eventual infection in this investigation. Furthermore, those who were vaccinated early in the immunization campaign had greater infection rates.
Taken together, these considerations suggest that the BNT162b2 vaccination, as recommended by the manufacturer, provides inferior long-term protection against infection, regardless of the SARS-CoV-2 variant type. These findings have influenced the decision to offer a third booster dose of the BNT162b2 vaccine after six months of receiving the second dose.