The coronavirus disease 2019 (COVID-19) pandemic was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several variants of which have emerged over the last year or so. The more infectious B.1.617.2 (delta) variant of the SARS-CoV-2 was responsible for an increase in cases across the globe until very recently.
A double dose of COVID-19 messenger RNA (mRNA) vaccines showed only modest differences in vaccine effectiveness between the delta variant and the original viral strain. Individuals with immunocompromising conditions, such as those undergoing solid organ transplantation (SOTR), may experience very different symptoms.
Furthermore, the recently spreading SARS-CoV-2 Omicron variant may further facilitate viral immune escape. Unfortunately, there has never been a comprehensive study of vaccine-induced antibody responses in SOTR that describes immunodominant antibody epitopes.
A new preprint research paper posted to the medRxiv* server describes the reduction in humoral immunity in SOTR following vaccination, as well as the improvement with an additional dose of the vaccine.
 Study: Humoral immune responses against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine. Image Credit: Skylines / Shutterstock
Study: Humoral immune responses against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine. Image Credit: Skylines / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The appearance of high titers of neutralizing antibodies to the virus after natural infection or vaccination, or both, is a correlate of protective immunity against reinfection or breakthrough infection, reducing the severity of the disease and limiting viral spread. Among SOTR, however, the humoral immune response is relatively less protective, and only about half of these patients develop an antibody response to the virus after infection.
Factors correlated with a reduced antibody response include kidney transplants, higher or more potent immunosuppression, and more recent transplant surgery history.
Three vaccines have received full approval or emergency use authorizations from the U.S. Food and Drug Administration (FDA). While all of them induce antibody- and cell-mediated immunity, with high degrees of protection against future infection for several months, the same is not true of SOTR. This group might be even more vulnerable to newer variants like the Delta and Omicron variants of concern (VOC) of SARS-CoV-2.
The current study aimed to describe vaccine-induced antibody responses in SOTR, and the results of a third booster dose of a messenger ribonucleic acid (mRNA) vaccine on humoral immunity against the different variants of the virus.
What Did the Study Show?
The study was performed with a prospective design on SOTR aged 18 years or more, who had received or were about to get any of the three vaccines. The vaccine recipients had received their first dose within 90 days of enrolment. None of the patients had human immunodeficiency virus (HIV) infection, were on chemotherapy or receiving radiation therapy, or had a history of infection with SARS-CoV-2.
The investigators measured the antibody levels to the viral proteins, to the S1 subunit of the spike protein, vs. viral control proteins. They also measured neutralizing antibody titers. The results showed significantly lower neutralizing antibody activity in SOTR after primary series with any of these vaccines.
In SOTR, antibody-mediated inhibition of binding between the viral receptor-binding domain (RBD) and host angiotensin-converting enzyme 2 (ACE2) receptor at 8 weeks from the second dose of an mRNA vaccine or 90 days from a single dose of the J&J vaccine was reduced.
While all controls showed over 90% neutralization of viral binding (“good responders”), this was found in less than one in five SOTR. Most SOTR, that is, 60% and 20%, were non-responders (less than 30% neutralization) and reduced responders (30-90% neutralization), respectively.
Specifically, the use of antimetabolites was linked to the most significant reduction in antibody-mediated viral neutralization. All SOTR groups had lower immunoglobulin G (IgG) antibody titers but comparable total IgM titers compared to controls. IgA levels were lower in good responders among SOTR.
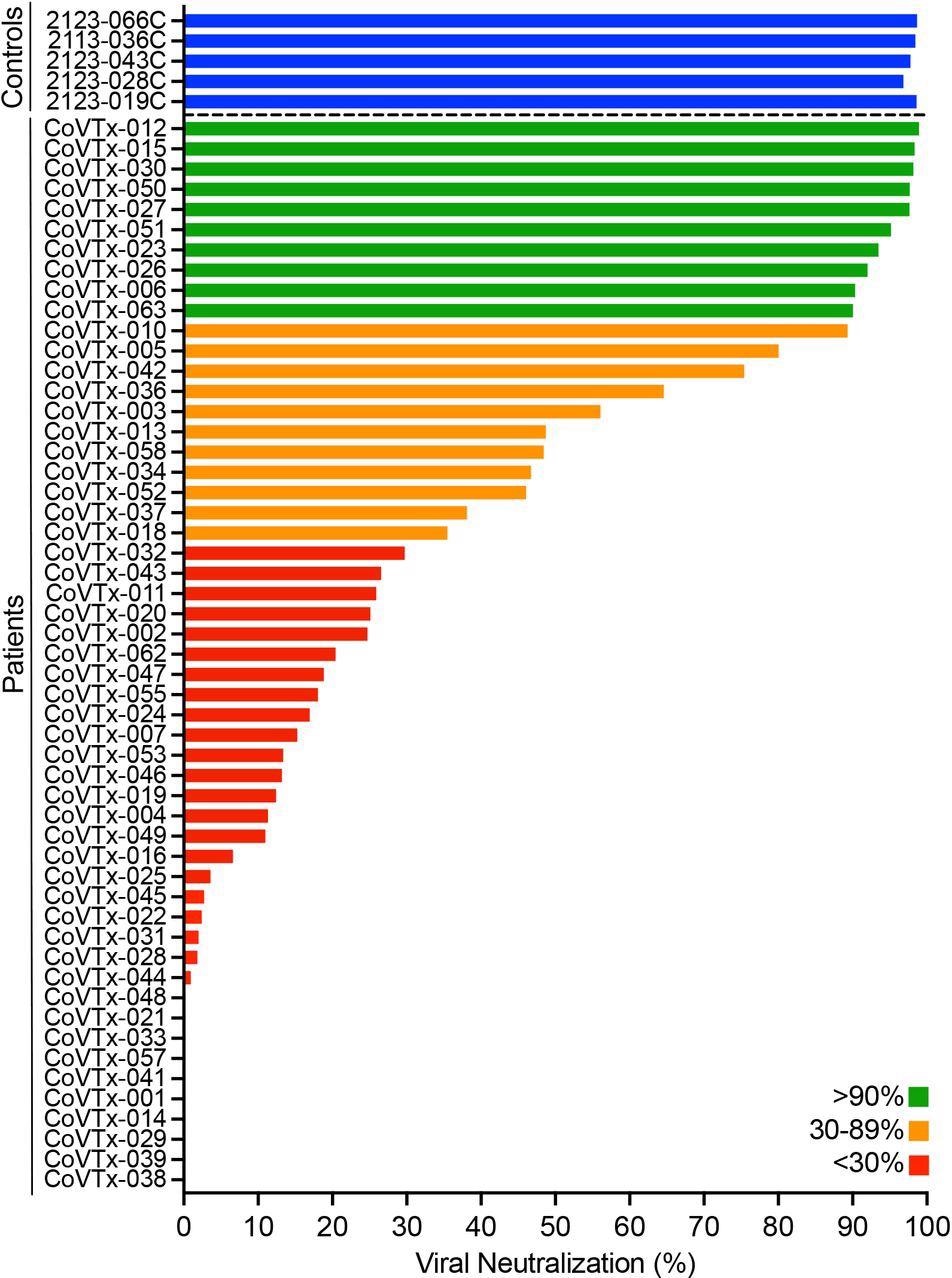
Neutralizing activity in the peripheral blood of SOT recipients after two doses of a COVID-19 mRNA vaccine Neutralizing activity of vaccine-induced anti-RBD antibodies in the peripheral blood of SOT recipients (N=53) and healthy controls (N=5; blue bars) after the second dose of the vaccine was measured as the degree of inhibition of RBD-ACE2 interactions. Green, orange, and red bars indicate different degrees of inhibition as indicated in the legend.
Vaccine-elicited antibodies showed lower diversity against SARS-CoV-2 spike and RBD antigens in SOTR compared to healthy controls. Among SOTR, even good responders showed lower antibody titers to these proteins in the Delta VOC.
IgA vaccine-induced antibodies to S1 and RBD were lower in all SOTR for all variants, with the exception of the good responder group, which showed high IgA responses to the S2 subunit of the spike protein, comparable to controls. The absence of antibodies to the nucleocapsid protein ruled out an infection-induced response.
The scientists also found that the titers of anti-RBD antibodies after a complete primary series with any of the vaccines were correlated to neutralizing activity against the virus, corroborating earlier studies. In addition, all patients with anti-RBD antibody titers above 4,500 also had over 90% neutralizing activity.
The reduced breadth of antibody response to viral epitopes in vaccinated SOTR indicates that linear epitopes targeted by B cells were restricted to a small region near the RBD C-terminal domain.
In SOTR, however, an additional vaccine dose caused a steep rise in neutralizing activity and antibody titers, irrespective of infecting variant, except for Omicron. The proportion of good responders to the wildtype RBD soared to 56% from the earlier level of less than 20%, while non-responders went down to 22%.
With the Delta and Alpha RBD variants, after an additional vaccine dose, good responders made up 60% in place of the earlier <1%. Non-responders now comprised 25% in place of 56% and 66% for the Delta and Alpha VOC, respectively. With the Beta VOC, there were no good responders with two doses, but an additional dose increased this group to 41% - which remains lower than for the other variants, however.
The median titers of the anti-RBD and anti-S1 antibody titers went down markedly, by 30% and 60%, respectively, with the Omicron VOC, compared to wildtype RBD.
What Are the Implications?
The results here show that the majority of SOTR show a poor response to primary vaccination against COVID-19, with less than one in five showing a high neutralizing response compared to healthy controls. While the IgG levels to SARS-CoV-2 antigens in such patients after one vaccine dose were lower than in controls, they were adequately or even more highly induced against cytomegalovirus and the flu virus, for instance, even in those with a poor anti-SARS-CoV-2 response.
This indicates the impaired production of humoral immunity against the SARS-CoV-2 spike protein, perhaps due to poor cellular response after the priming dose or due to restricted expansion of the B cells. In fact, IgG titers to the S1 and RBD antigens are reduced following vaccination in SOTR patients who have a lower overall neutralizing activity against the virus. Simultaneously, the results supported the excellent correlation of anti-RBD antibody titers with viral neutralization.
The finding that antimetabolite treatment was associated with the most significant reduction in the humoral response to the vaccines confirms the results of earlier studies with these vaccines as well as with antibodies to the seasonal flu virus in patients being treated with drugs like mycophenolate mofetil (MMF), a prodrug of mycophenolic acid (MPA). Not only the rates of seroconversion but of activated CD4+ T cells against the H1N1 flu virus were reduced in these patients.
This is due to the inhibition of inosine-50-monophosphate dehydrogenase (IMPDH) by MPA. This prevents the formation of certain nucleotides and thus inhibits nucleic acid replication. This reduces the ability of B and T cells to proliferate and produce antibodies. In vivo studies have shown the drug also impairs the antibody response to priming antigens, though not booster antigens.
Thus, in patients taking MMF after a kidney transplant or due to systemic lupus erythematosus (SLE), seroconversion has been shown to be reduced.
“Future studies should evaluate in detail the effect of MMF intake on B cell and T cell phenotype and function after COVID-19 vaccination to help improve immune responses in SOTR.”
The study also showed that most antibodies elicited by the vaccine targeted a small region near the C-terminal domain of the RBD in SOTR. This immunodominant region may explain why antibody titers are low and immune escape so common in this group.
Encouragingly, an additional vaccine dose caused dramatically improved antibody responses in these patients, enhancing the total antibody titer and the neutralizing activity. The improvement was most marked for the Delta and Alpha VOC, compared to the Beta. This is an important caveat when it is remembered that the Omicron shares several immune escape mutations with the latter VOC.
In fact, IgG anti-S1 and anti-RBD antibody binding to the wildtype viral proteins was much higher compared to the Omicron variants. This could indicate a drastic drop in vaccine-induced immune protection conferred by the primary vaccine series in SOTR. This should stimulate research into the use of additional or booster doses of vaccine or pre- or post-exposure prophylaxis in this group of patients who are at high risk for mortality following SARS-CoV-2 infection.
In fact, immunocompromised patients are particularly fertile ground for the emergence of new variants of the virus with multiple mutations, due to the prolonged state of replicative infection in this group.
“This is another important reason to find ways to prevent these individuals from becoming infected or at least limit the duration of viral persistence in case of an active COVID-19 infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources