Memory B cells never forget recurrent viral exposures and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is no exception, according to a new medRxiv* preprint study. Vaccination and prior infection appear to activate long-lived memory B cells and mold them to become receptor-binding domain (RBD)-specific B cells. When given a third vaccine dose, pre-existing memory B cells became cross-reactive and successfully bound Omicron’s spike protein.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Characterizing memory and effector B cell subsets
During the study, researchers from various institutions across the U.S. and South Africa examined fresh blood samples from a small group of unvaccinated and uninfected subjects prior to vaccination and 1-2 weeks following vaccination with an mRNA vaccine (BNT162b2 or mRNA-1273, respectively) (Post Vax).
Two spike-specific tetramer probes were generated to identify and categorize memory and effector B cell subsets after vaccination. There were 12 identified clusters, but clusters 7 and 9 were classified as being CD27 high or low, making 14 clusters in total.
Effector B cell subsets and switched memory B cell subsets could be found through their expression of specific surface proteins such as CD21, CXCR5, CD11c, and CD85j.
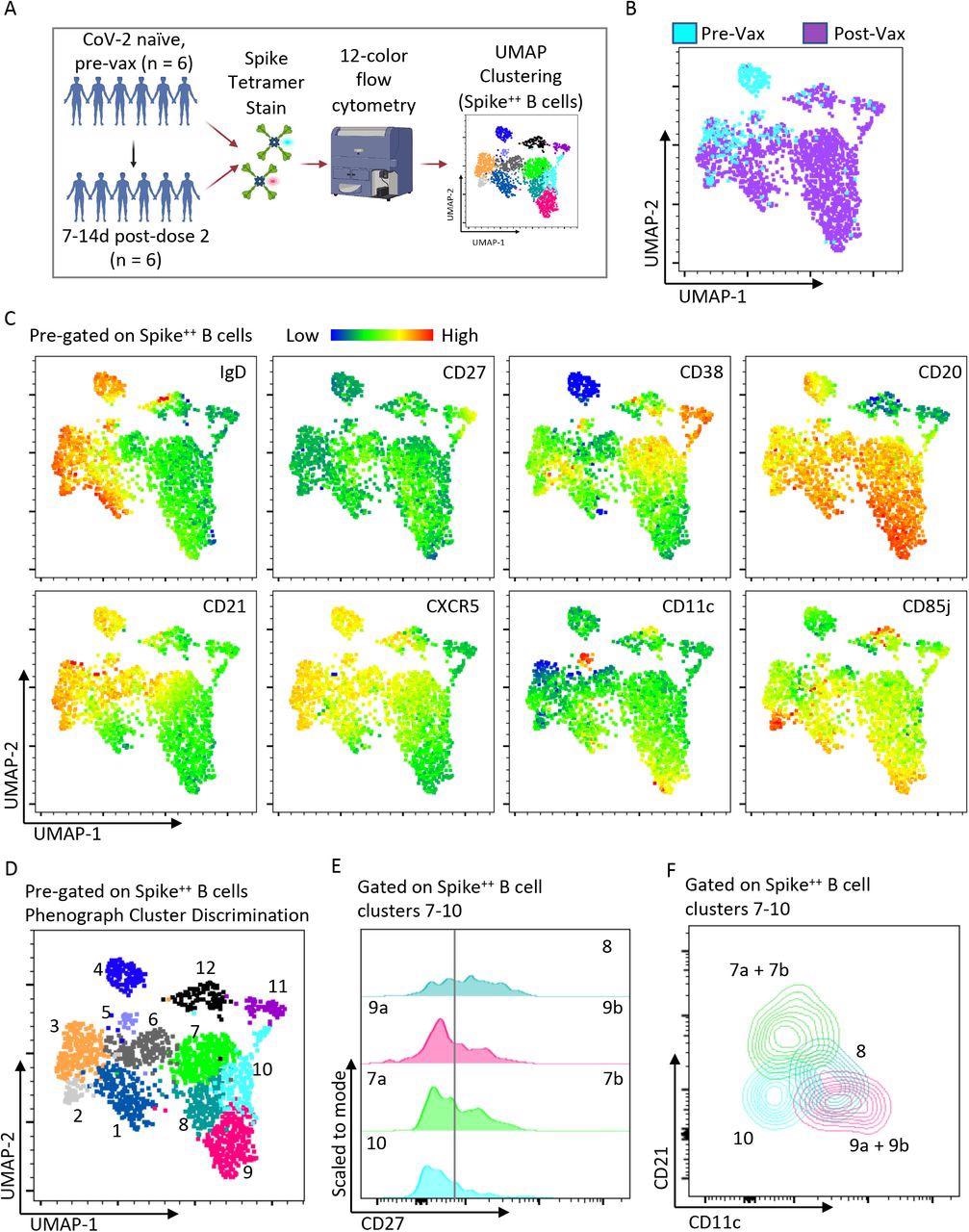
Heterogeneous spike-specific B cells emerge following mRNA vaccination. A) Pictorial overview of initial flow cytometry studies using spike-specific probes and unbiased clustering. B) UMAP dimensionality reduction displaying all Spike-specific B cells from previously unexposed, healthy individuals (n=6) and paired post-vaccination dose #2 (n=6) from the same individuals. The plot is color coordinated based on timepoints of pre-vaccination (Pre-Vax) and post-vaccination (Post-Vax). C) Heatmap overlays of the same UMAP from figure B displaying the relative expression patterns of IgD, CD27, CD38, CD20, CD21, CXCR5, CD11c, and CD85j. D) Overlay of color-coordinated clusters among Spike-specific B cells discriminated using Phenograph. E) Histograms displaying relative contributions from double-negative B cells (CD27Negative) and switched memory B cells (CD27+) among Phenograph clusters 7 through 10. F) Overlaid contour plots of clusters 7 through 10 displaying a relative expression of CD21 and CD11c.
Memory B cell expansion after vaccination and COVID-19 infection
In people who were later infected with SARS-CoV-2 and vaccinated, the team noticed the presence of spike-binding circulating B cells that would usually stick around for at least 2 to 3 months after vaccination. The induction of spike-binding circulating B cells was associated with a CD27+ SWM-type phenotypic change among spike-binding B cells compared to B cells before vaccination.
Of all the changes, vaccination-induced the expansion of IgDCD85j+CD27+ B cells. The B cell expansion persisted months after vaccination. IgDCD85j+CD27+ B cells were also seen in patients in the convalescent phase of COVID-19.
IgDCD85j+CD27+ B cells were not found in plasma samples of uninfected individuals and not vaccinated. However, the researchers note that these cells do not last for more than six months after vaccination, suggesting they are more effector B cells than memory B cells.
Another group of B cells, IgD- CD27+ CD85j- CD38int, expanded the most after vaccination and infection.
Changes to pre-existing resting memory B cells
Uninfected and unvaccinated individuals showed high levels of pre-existing spike binding B cells that target SARS-CoV-2. However, exposure to only other seasonal coronaviruses makes it harder to recognize the spike proteins of other coronaviruses such as SARS-CoV-2.
A large number of spike-binding B cells are found in the IgD+CD27- “naive” gate as well as the unswitched memory population, “switched memory” B cell gate, and the IgDCD27- switched CD27- B cell population.
The majority of spike-binding B cells targeting SARS-CoV-2 and the Omicron variant are comprised of naive follicular B cells. Unvaccinated and uninfected individuals lack RBD-binding B cells.
After vaccination or infection, the number of spike-binding resting naive follicular B cells decreased, suggesting an expansion of other B cells and potential activation of spike-binding naive follicular B cells on an as-needed basis.
A small percentage of spike-binding B cells are resting unswitched memory B cells made up of marginal zone B cells. However, the proportion of marginal zone B cells is not reduced after infection and vaccination, suggesting limited or no activation in this naive B cell population.
Infection and vaccination also caused the decline of pre-existing resting CD27+ switched memory B cell compartment. However, pre-existing CD27-negative switched memory B cell compartments of spike-binding proteins further expanded after vaccination and infection.
Compared to receptor-binding B cells targeting the wild-type SARS-CoV-2, the pre-existing CD27-resting switched memory B cell compartment is one of the most dominant and long-lasting antigen-specific memory B cell populations in vaccinated individuals six months after vaccination but before a booster shot.
Cross-reactivity of B cell memory populations against Omicron
Six months after vaccination caused a majority of pre-existing memory B cells to morph into RBD-binding memory B cells. The researchers also observed that RBD-binding B cells were the most dominant among vaccinated individuals.
Given the rise of Omicron-related COVID-19 cases, the researchers investigated how booster shots affect memory B cell’s actions against the new variant of concern.
Boosting did not increase the frequency of RBD-binding B cells that recognize Omicron’s RBD. However, it did induce differentiation and expansion of pre-existing switched memory B cells such as CD27+ and CD27- which held bind Omicron’s RBD. Additionally, a booster increased the frequency of wild-type spike-binding B cells that expand to target and bind Omicron.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.