
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Studies have shown that newly emerging variants of SARS-CoV-2 can escape natural and vaccine-induced antibodies and monoclonal antibody immunotherapeutics. In the United Kingdom, the AstraZeneca (AZ) ChAdOx1 nCoV-19 vaccine and Pfizer-BioNTech (PFZ) BNT162b2 vaccine were the most administered vaccines.
The effectiveness of both the AZ and PFZ vaccines in reducing the transmission and severity of SARS-CoV-2 infection caused by the currently dominant Delta and Omicron VOCs in the general population and immunologically vulnerable groups is not well understood.
About the study
In the present study, the researchers compared the cross-reactive binding of vaccine-induced antibodies in serum samples collected from three different cohorts at six months post second dose and 28 days after the third dose.
Among the three cohorts, one cohort included convalescent healthcare workers (COCO) who received three doses of Pfizer-BioNTech (PPP), the second cohort included Clinically Extremely Vulnerable (CEV) patients who received two doses of the AstraZeneca-ChAdOx1-nCoV-19 (AAP) vaccine followed by a third PFZ vaccine. The third cohort included individuals requiring hemodialysis (HD) who had received a mixture of two AZ or PFZ vaccines followed by a PFZ booster dose.
The researchers used enzyme-linked immunoassay (ELISA) to measure the immunoglobulin G (IgG) antibodies specific to the spike protein of the original Wuhan strain of SARS-CoV-2, as well as the Delta (B.1.617.2) and Omicron (B.1.1.529) variants.
Demographic observations
The median age was 47 years in the COCO cohort, and 21% of subjects were male. The health care workers of this cohort received all three doses of the Pfizer-BioNTech vaccine (PPP). A total of 90 serum samples before the third dose and 60 serum samples after the third dose were tested.
In the CEV cohort, the median age was 51 years had 44% of the subjects were male. The subjects of this cohort received two doses of the AstraZeneca-ChAdOx1-nCoV-19 vaccine and a third dose of the PFZ vaccine. A total of 24 serum samples before the third dose and 47 serum samples after the third dose were tested.
The median age was 66 years in the HD cohort, with the male gender accounting for 61% of the subjects. Among this group, 70.3% received AZ vaccine as the primary course, with the rest of the subjects receiving PFZ. A total of 90 serum samples before the third dose and 85 serum samples after the third dose were tested.
Study findings
ELISA of serum samples collected six months after the second vaccine dose revealed that 58.9%, 34.4%, and 62.2% of individuals tested positive for the Wuhan, Delta, and Omicron strains in the HD cohort, respectively. In the COCO cohort, 92.2%, 90%, and 91.1% tested positive for the Wuhan, Delta, and Omicron strains, respectively. In the CEV cohort, 62.5% were positive for the Wuhan strain, 48.8% for the Delta strain, and 91.7% for the Omicron variant.
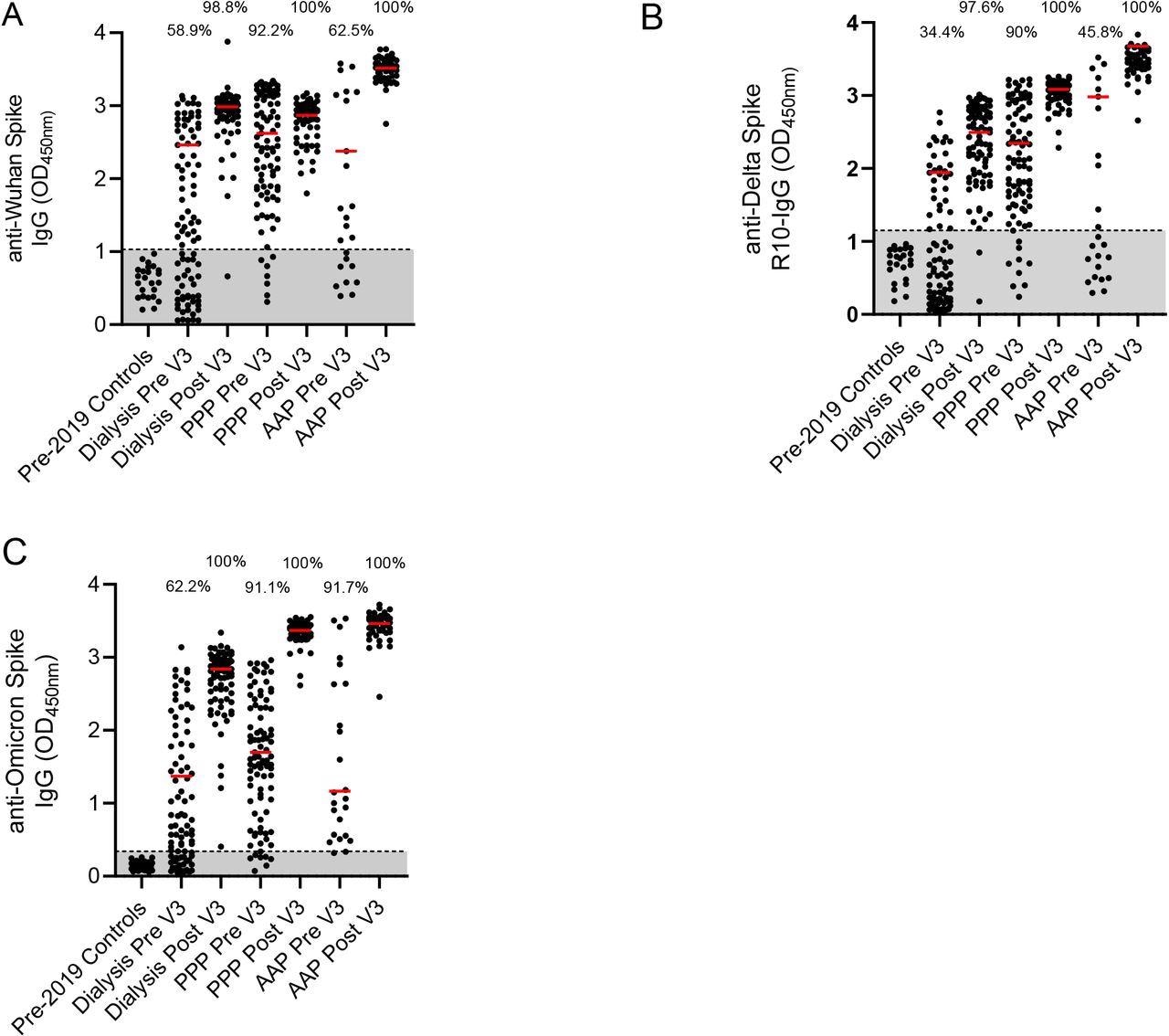
Percentage of the cohort with antibodies against Wuhan, Delta and Omicron strains A – Detection of anti-Wuhan spike IgG in pre-2019 controls, a dialysis population, a cohort of health care workers who have had three PFZ vaccines, and a Clinically Extremely Vulnerable population in general practice who have had two AZ and one PFZ vaccine. Results are given for pre and post 3rd doses of vaccination. Percentage of the cohort that is considered seropositive are included above the dot plots. The red line represents the median of the seropositive individuals in that cohort. B – Detection of anti-Delta for the same populations. C – Detection of anti-Omicron for the same populations Pre – pre 3rd dose of vaccine and 6 months post 2nd dose. Post – 28 days post 3rd dose of vaccine. PPP – 3 Pfizer-BioNTech vaccines given in this cohort. AAP – two AstraZeneca ChAdOx1 nCoV-19 vaccines and the one Pfizer-BioNTech vaccine given in this cohort
Further, ELISA results of serum samples collected post 28 days after the third dose provided information on the antibody concentration as median optical density (OD). This assay revealed a significant increase in median OD of seropositive individuals of all cohorts against spike proteins from viral VOC.
The HD cohort showed a significant increase in seropositivity, with 98.8% for the Wuhan strain, 97.6% for the Delta variant, and 100% for the Omicron variant. The COCO and CEV cohorts displayed 100% seropositivity against all three strains. The researchers compared the median OD of the COCO and CEV cohorts. They found that the CEV cohort had consistently high antibody concentration against the Wuhan and Delta strains but not the Omicron variant.
The researchers also examined the World Health Organization (WHO) National Institute for Biological Standards and Control (NIBSC) 20/136 against the Wuhan, Delta, and Omicron spike glycoproteins using ELISA. To this end, they found no loss of antibody binding to either SARS-CoV-2 VOC.
Likewise, a dilution series of 6.5 mg/ml to 0.003 mg/ml Sotrovimab (GSK) revealed that this therapeutic monoclonal antibody binds similarly to the Omicron spike glycoprotein as to the other spike glycoproteins.
Conclusions
Overall, the study findings demonstrate that the vaccine-induced antibody response wanes six months after receiving the second dose. Therefore, a third dose of the vaccine is essential to maintain the protective response against SARS-CoV-2, as it can provide cross-reactive humoral immunogenicity against rapidly emerging SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Faustini, S. E., Shields, A. M., Banham, G., et al. (2021). Crossreactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose. medRxiv. doi:10.1101/2021.12.30.21268308, https://www.medrxiv.org/content/10.1101/2021.12.30.21268308v1
- Peer reviewed and published scientific report.
Faustini, Sian E, Adrian M Shields, Gemma D Banham, Nadezhda A Wall, Saly Al-Taei, Chloe Tanner, Zahra S Ahmed, et al. 2022. “Cross Reactivity of Spike Glycoprotein Induced Antibody against Delta and Omicron Variants before and after Third SARS-CoV-2 Vaccine Dose in Healthy and Immunocompromised Individuals.” Journal of Infection 84 (4): 579–613. https://doi.org/10.1016/j.jinf.2022.01.002. https://linkinghub.elsevier.com/retrieve/pii/S0163445322000020.