
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Currently, anti-SARS-CoV-2 mAbs are used to induce active immunity against coronavirus disease 2019 (COVID-19) in immunocompromised patients who were unresponsive to the complete vaccine regimen. Studies have shown that different strains of SARS-CoV-2 have variations in susceptibility towards mAbs. Moreover, detailed information regarding mAb-associated neutralization of the SARS-CoV-2 Omicron variant is not yet available.
About the study
In the present study, researchers tested the neutralizing activity of mAbs such as bamlanivimab, etesevimab, casirivimab, and imdevimab against SARS-CoV-2 wild-type (B.1.1), B.1.160, Iota (B.1.526), Mu (B.1.621), Alpha (B.1.1.7), Beta (B.1.351.2), original Delta (AY.71), Delta sublineage (AY.4.2), Epsilon (B.1.429), and Omicron (B.1.1.529) strains.
Vero E6 cells were cultured without antibiotics in a minimum essential medium (MEM) and then used for the neutralization tests of SARS-CoV-2 in MEM growth medium with fetal bovine serum (FBS) and glutamine.
The ten SARS-CoV-2 strains used in this study were isolated from SARS-CoV-2-positive nasopharyngeal swabs in cell culture and stored at -80°C in IHU Méditerranée Infection institute. Later, the supernatant of each strain was harvested and genotyped using whole-genome next-generation (NGS) sequencing technology.
Neutralization tests were carried out by inoculating the viral strains into Vero E6 cells. Two days after viral infection, the suspension was quantified using reverse transcription-polymerase chain reaction (RT-PCR) and median tissue culture infectious dose (TCID50) assay.
All mAbs used in the study including etesevimab, bamlavinimab, imdevimab, and casirivimab were diluted in 1:5 serial dilutions. For the combination of etesevimab + bamlavinimab and casirivimab + imdevimab, the highest concentration of the mixtures was tested against two times more etesevimab than bamlavinimab and two times more casirivimab than imdevimab mixtures, respectively.
A microneutralization assay was conducted by mixing mAb dilutions with each viral strain. The mAb titer required to obtain 50% neutralization against SARS-CoV-2 variants was determined using an inverted optical microscope five days after viral infection. Moreover, mAbs and their combinations were tested three times against all SARS-CoV-2 variants, except Omicron, which was tested four times.
Study findings
Bamlavinimab did not inhibit the SARS-CoV-2 Mu, Epsilon, Delta, and Beta variants. Etesevimab showed 50% neutralization below 5 µg/mL of the SARS-CoV-2 Epsilon, wild-type, and both Delta variants. In etesevimab and bamlanivimab combinations, significant neutralization was detected against the B.1.160, Iota, and Alpha strains.
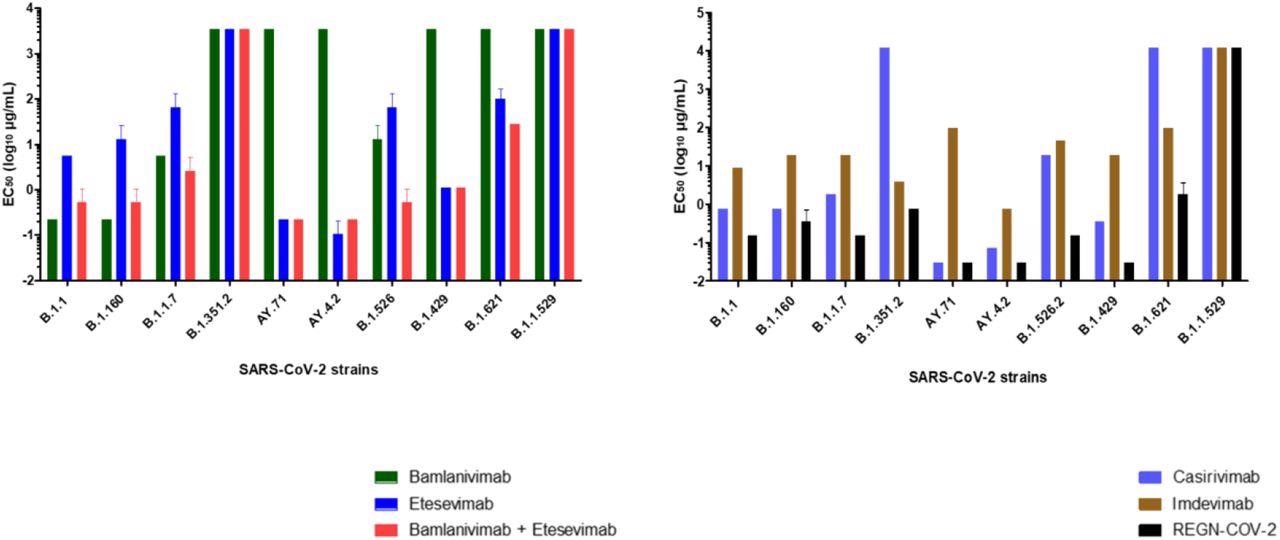
Concentrations required obtaining 50% neutralization (EC50 log10 µg/mL) for each mAb. (A) bamlanivimab, etesevimab, mixture of bamlanivimab and etesevimab, (B) casirivimab, imdevimab and REGN-CoV-2 on the 10 SARS-CoV-2 strains tested. Each mAb was tested three times (except for Omicron variant 4 times).
Casirivimab showed remarkable neutralization against the B.1.160, wild-type, Alpha, Iota, Epsilon, and both Delta variants of SARS-CoV-2. In contrast, casirivimab did not neutralize SARS-CoV-2 Beta and Mu variants.
Imdevimab neutralized all SARS-CoV-2 variants except Omicron. However, imdevimab concentrations required for 50% neutralization of SARS-CoV-2 variants were more than that of casirivimab.
The casirivimab + imdevimab combination showed a synergistic effect, especially against the Epsilon, AY4.2, and AY.71 variants, with 50% neutralization at 0.03 µg/mL concentration. The casirivimab + imdevimab combination showed 50% neutralization at 0.2, 0.4, and 0.7 µg/mL concentrations against the SARS-CoV-2 Original, Iota, Alpha, and B.1.160, and Beta variants, respectively.
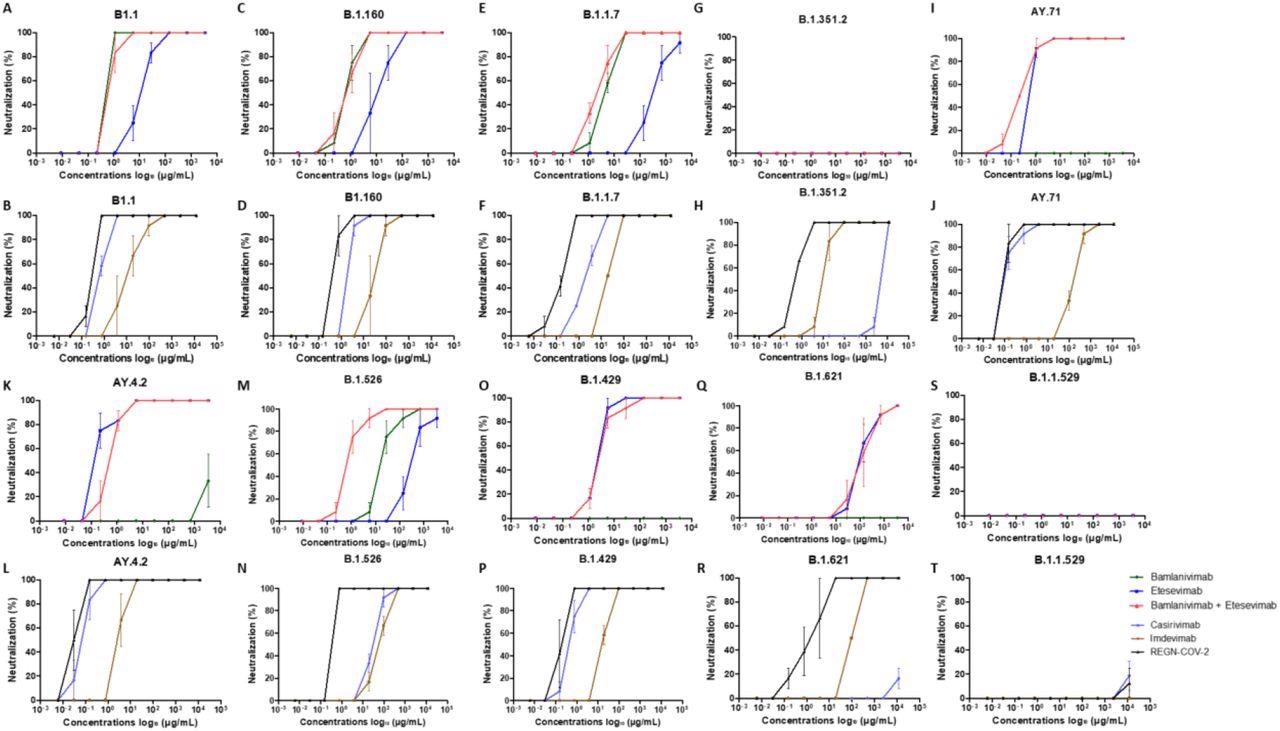
Neutralization curves in Vero E6 cells for each strains tested with each mAb : A, C, E, G, I, K, M, N, O, Q, S : bamlanivimab, etesevimab and mixture of bamlanivimab and etesevimab – B, D, F, H, J, L, N, P, R, T : casirivimab, imdevimab and REGN-CoV-2. Each experiment was done three times, except for the Omicron variant four times.
At a concentration of 2 µg/mL, the casirivimab + imdevimab combination showed 50% neutralization against the SARS-CoV-2 Mu variant. However, none of the four mAbs alone or in combination exhibited neutralizing capacity against Omicron.
Conclusions
The study findings suggest that four mAbs tested had a lower inhibitory effect on recently emerging SARS-CoV-2 variants. However, their combination was highly effective, particularly against the Delta variant. In contrast, the four mAbs combined or alone were ineffective against the Omicron variant.
The present study underscores the reinforcement of protective measures against SARS-CoV-2 Omicron infection among immunocompromised patients. However, further studies are required to confirm the ineffectiveness of mAbs against the Omicron variant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Boschi, C., Colson, P., Bancod, A., et al. (2021). Omicron variant escapes therapeutic mAbs contrary to eight prior main VOC. bioRxiv. doi:10.1101/2022.01.03.474769. https://www.biorxiv.org/content/10.1101/2022.01.03.474769v1.
- Peer reviewed and published scientific report.
Boschi, Céline, Philippe Colson, Audrey Bancod, Valérie Moal, and Bernard La Scola. 2022. “Omicron Variant Escapes Therapeutic MAbs Including Recently Released Evusheld ®, Contrary to Eight Prior Main VOC.” Clinical Infectious Diseases, February. https://doi.org/10.1093/cid/ciac143. https://academic.oup.com/cid/article/75/1/e534/6529556.