
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Some DMTs for MS treatment may lead to a diminished immune response in patients presenting with coronavirus disease 2019 (COVID-19). Furthermore, the anti-CD20 therapies (aCD20) commonly used for MS patients have been reportedly associated with lower antibody titers in COVID-19-infected and vaccinated individuals.
Although studies have shown that aCD20 DMTs result in depleting numbers of peripheral B-cells, T-cells are relatively unaffected, as only a small subset of CD20-expressing T-lymphocytes are eliminated by aCD20 therapies.
Studies have shown that the cellular response remains intact in aCD20 recipients. Moreover, the antigen-specific T cell response to SARS-CoV-2 in vaccinated individuals is unaffected and remains robust; however, the response in infected individuals has yet to be determined.
Similarly, more research is required to investigate the impact of other DMTs like sphingosine-1-phosphate receptor modulators (S1P), which likely intervene with T-cell egress from lymphoid tissues and fumarates known to induce mild-to-moderate lymphopenia.
About the study
The present study addressed the impact of ocrelizumab (OCR) and other DMTs on cellular and humoral immune memory in SARS-CoV-2-infected individuals. The researchers also characterized the relationship between humoral and cellular immunity in infected individuals with and without B cell depletion. They assessed the clinical severity of COVID-19 and host-immune response to its etiologic agent in MS patients with different DMTs.
The current study included 389 non-vaccinated MS patients between the ages of 18 and 60 years from the New York University (NYU) MS Care Center. These patients were ethnically diverse, with more than 60% of participants being non-white and about 74% of females.
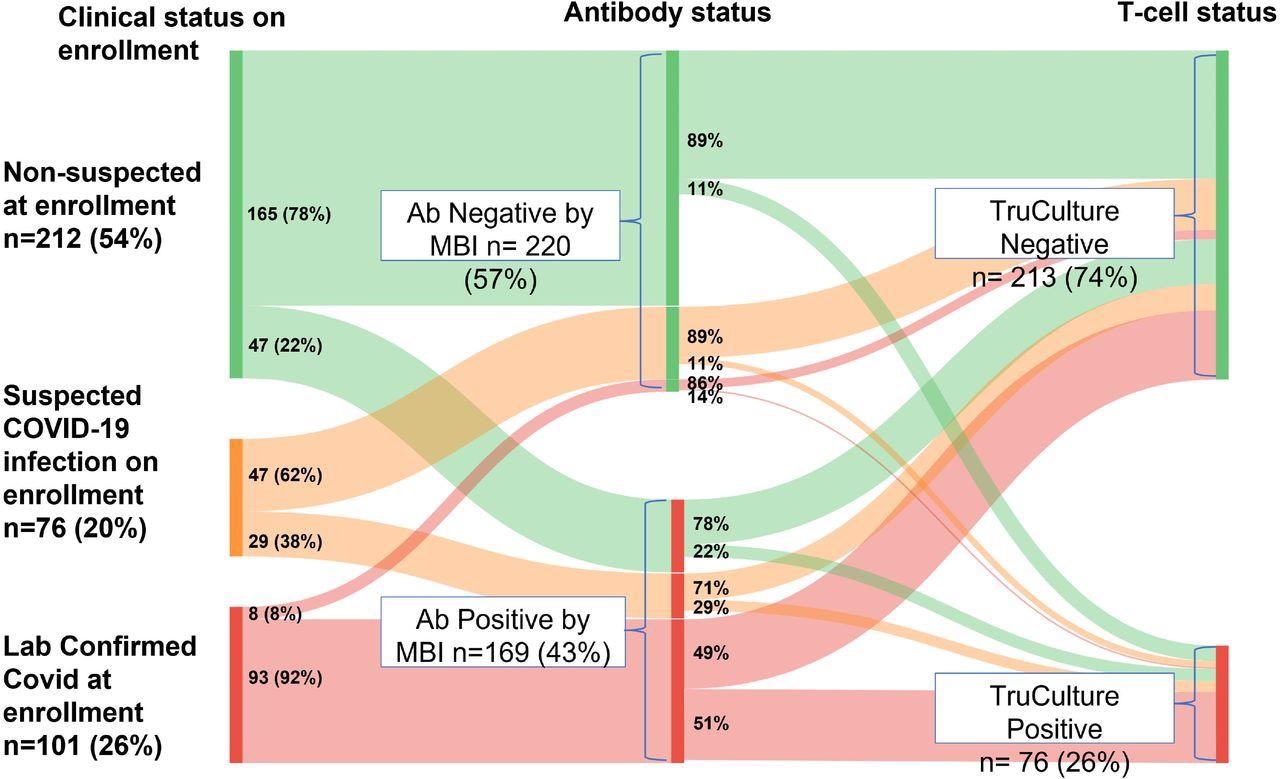
COVID-19 history at enrollment (left panel) stratified by MBI serostatus (middle) and TruCulture (right) Legend: Sankey diagram illustrates proportions of patients with ‘laboratory-supported COVID at enrollment’, ‘suspected COVID at enrollment’ and ‘COVID not suspected at enrollment’ who tested positive by MBI (middle panel) and proportion of MBI-positive and MBI-negative patients who tested positive on Truculture (see Section 6). ‘Lab-supported COVID on enrollment’ is defined as ‘clinical symptoms consistent with COVID-19 (CDC clinical case definition) and laboratory confirmation of SARS-CoV-2 prior to enrollment’. ‘Suspected COVID on enrollment’ is defined as ‘clinical symptoms consistent with COVID-19 (CDC clinical case definition) but no laboratory confirmation of SARS-CoV-2 before enrollment’. ‘Not suspected on enrollment’ is defined as not meeting CDC clinical case definition. ‘MBI seropositive’ is defined as ‘≥2/3 independent antibody assays >3SD over the mean pre-pandemic levels. ‘TruCulture positive’ was defined as both IFNγ and IL-2 assays were at the level of ≥1 pg/ml.
The patients were assessed for the presence of antibodies to SARS-CoV-2 spike (S) receptor-binding domain (RBD) and nucleocapsid (N) protein using an electro-chemiluminescence immunoassay (ECI) and to the whole S protein, S RBD, and N-terminal domain (NTD) of S protein using a multiepitope bead-based immunoassay (MBI).
A live-virus neutralization assay was performed on all patients. SARS-CoV-2-specific T-cell responses were evaluated with the TruCulture stimulation system, as well as the interferon- γ (IFN-γ) and interleukin (IL)-2 ELISpot assays. All assay results were stratified by different DMT classes.
Study findings
The authors noted that SARS-CoV-2 antibody responses were not racially differential by either method of immunoassays. In addition, a strong correlation was observed between individual assays by MBI for the whole S and NTD components of S and between anti-RBD antibody levels by MBI and ECI.
A 100% seropositivity rate was observed for patients with prior SARS-CoV-2 infection for all DMTs except OCR (89%) by MBI. Anti-S antibodies, as assayed by ECI and MBI, revealed that in OCR patients with prior SARS-CoV-2 infection, a 10-fold reduction was observed when compared to untreated patients with prior infection. Similarly, S1P patients also demonstrated lower antibody levels as compared to untreated patients as measured by ECI.
T-cell responses were positive for both IFN-γ and IL-2 assays in 39% of all patients with prior infection. Over 59% of the samples tested positive on both TruCulture and ELISpot assays. The concordant negative rate for IFN-γ was 15.7%, and the discordant rate was 24.7%. For IL-2 responses, concordant positive and negative rates were 36.9% and 33.3%, respectively.
Consistent with the humoral response results, no ethnically differential cellular responses were noted by either TruCulture or ELISpot assays. The authors also evaluated cellular responses in 130 SARS-CoV-2 seronegative patients by MBI and observed that 14 (11%) patients showed TruCulture reactivity. Of these 14 patients, none were seropositive as tested by ECI; however, nine exhibited seropositivity for NTD by MBI, and only 2 out of 4 samples showed positive ELISpot results.
DMT-stratified analysis revealed that the OCR group had similar TruCulture IFN-γ responses as compared with the untreated reference group, and the S1P and Natalizumab groups showed depressed and elevated responses, respectively. The IL-2 responses for Natalizumab recipients were slightly elevated, whereas these responses for S1P patients were marginally lower as compared to those for the untreated reference group.
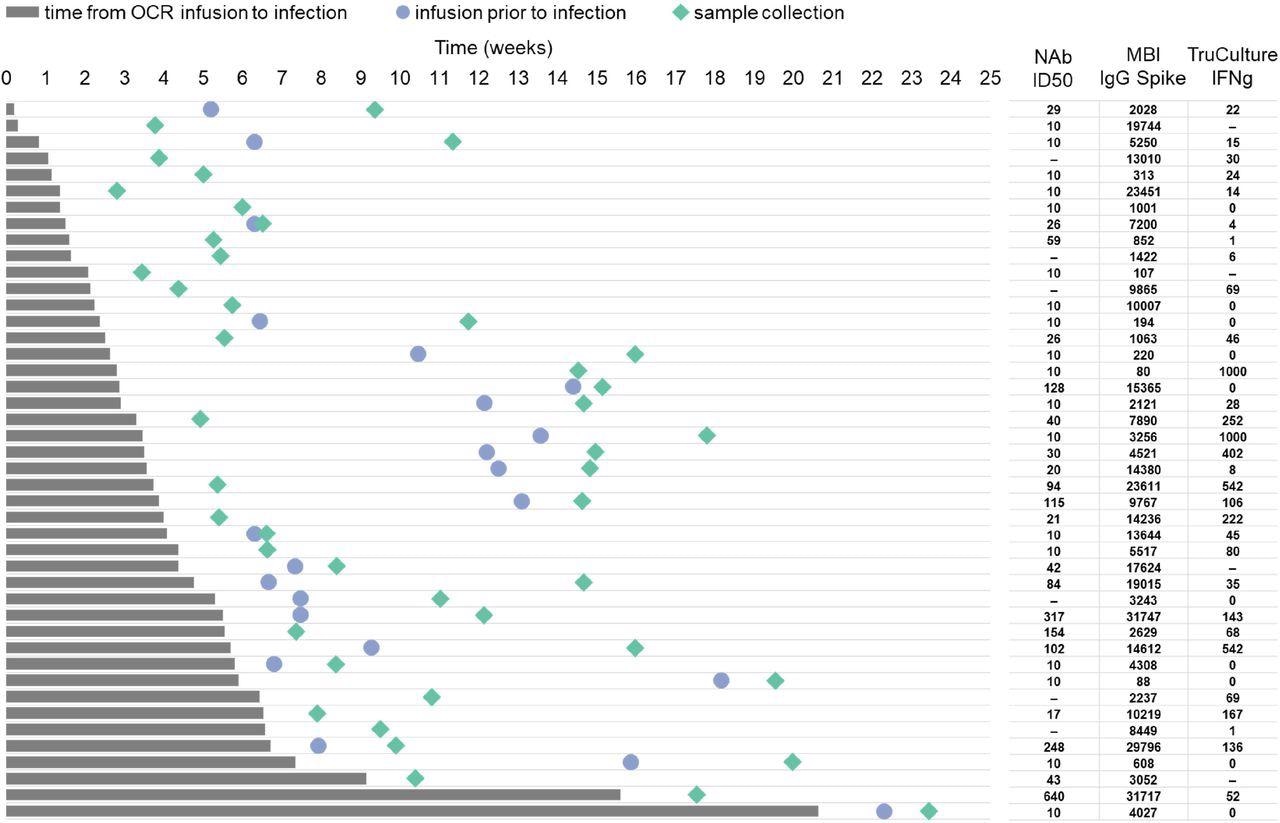
Timeline for symptomatic COVID-19 patients who were infected following OCR infusion Legend: Timeline from last OCR infusion before infection (time zero, start of the grey bar) to COVID-19 infection onset (end of the grey bar), subsequent OCR infusion before sample collection (blue circle) and sample collection (green rhombus). Each line represents a patient timeline. Neutralizing Ab titers, binding IgG anti-spike level by MBI and TruCulture IFNγ are shown for each patient in the respective line. Neutralizing antibody titers are shown as log10 of half-maximal inhibitory dilution (ID50).
Conclusions
The observations made in the study noted that the humoral response was markedly lower in OCR-treated patients compared to other DMT groups, the T-cell response increased marginally in S1P-treated patients, and a slight decrease was seen in Natalizumab patients when compared with reference groups.
MS patients on OCR and other DMTs showed similar cellular responses as the untreated patients and both the cellular and humoral responses remained similar in all ethnic groups. While the COVID-19 clinical course remained mostly non-severe in MS patients across all DMTs, the researchers observed no correlation in the clinical severity of COVID-19 and immune responses in MS patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kister, I., Yury, P., Curtin, R., et al. (2022). Cellular and Humoral Immunity to SARS-CoV-2 Infection in Multiple Sclerosis Patients on Ocrelizumab and Other Disease-Modifying Therapies: A Multi-Ethnic Observational Study. medRxiv. doi:10.1101/2022.01.10.22268752. https://www.medrxiv.org/content/10.1101/2022.01.10.22268752v1.full-text.
- Peer reviewed and published scientific report.
Kister, Ilya, Yury Patskovsky, Ryan Curtin, Jinglan Pei, Katherine Perdomo, Zoe Rimler, Iryna Voloshyna, et al. 2022. “Cellular and Humoral Immunity to SARS-CoV-2 Infection in Multiple Sclerosis Patients on Ocrelizumab and Other Disease-Modifying Therapies: A Multi-Ethnic Observational Study.” Annals of Neurology 91 (6): 782–95. https://doi.org/10.1002/ana.26346. https://onlinelibrary.wiley.com/doi/10.1002/ana.26346.