Scientists have worked at an unprecedented speed to develop vaccines to fight against the ongoing coronavirus disease 2019 (COVID-19) pandemic. This pandemic has been caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an RNA virus belonging to the family Coronaviridae.
 Study: Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. Image Credit: NIAID
Study: Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. Image Credit: NIAID
Background
It is imperative to understand the impact of COVID-19 vaccination on severe illness and hospitalization among children. This would help policymakers formulate better COVID-19 vaccination strategies to protect children from SARS-CoV-2 infection. In May 2021, the U.S. Food and Drug Administration (FDA) extended the emergency use authorization of the mRNA- based BNT162b2 COVID-19 vaccine, developed by Pfizer–BioNTech, to include adolescents aged between 12 and 15 years.
A randomized, placebo-controlled trial of the BNT162b2 vaccine revealed vaccine efficacy of 100% against symptomatic COVID-19 among adolescents. However, this trial lacked information about the effect of the vaccine against severe SARS-CoV-2 in adolescents. This is due mainly to the rare nature of this outcome. In early September 2021, pediatric hospitalization caused by the SARS-CoV-2 Delta variant pointed out the importance of evaluating the effectiveness of the BNT162b2 vaccine against severe COVID-19 in adolescents. Furthermore, a surge in the number of COVID-19 cases among adolescents presented an opportunity to determine the efficacy of BNT162b2 in real-world settings.
A New Study
A recent study revealed the effectiveness of 93% for the BNT162b2 vaccine against COVID-19 hospitalization among adolescents (12 to 18 years). This study included 179 patients at 19 sites in 16 states. The same group of researchers have extended their analysis to 31 locations in 23 states across the U.S. and recruited 266 more patients who had been hospitalized with SARS-CoV-2 infection.
This study has been published in the New England Journal of Medicine. In this study, scientists have significantly increased the sample size and analyzed the effectiveness of the BNT162b2 vaccine among SARS-CoV-2 infected adolescents in terms of intensive care unit (ICU) admission or requirement of other life-supporting interventions.
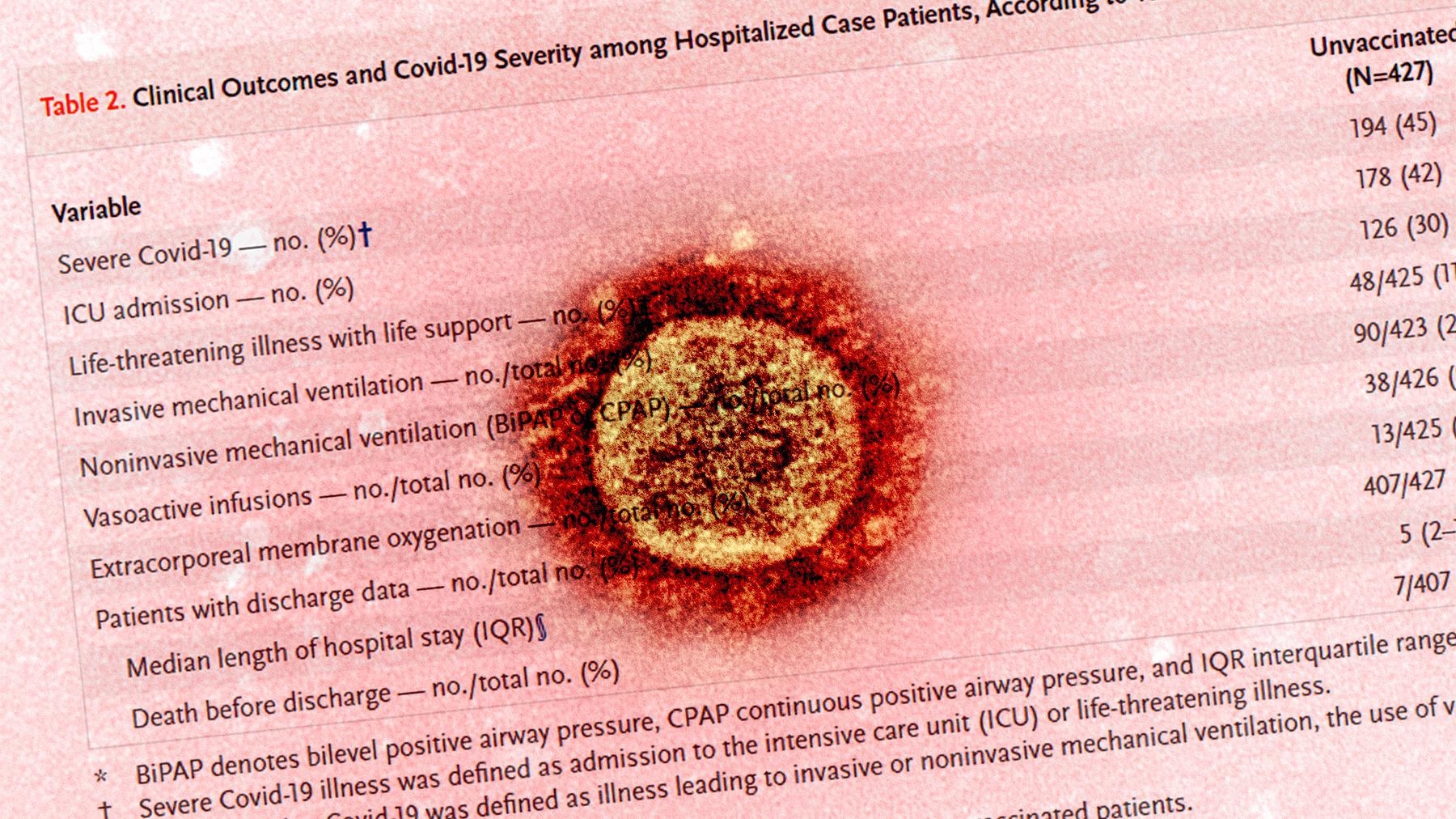
The study compared 445 adolescent COVID-19 patients who were hospitalized with 777 control patients without SARS-CoV-2 infection. Researchers reported that despite their eligibility, 96% of the patients who were hospitalized and 99% of those who received life support were not fully vaccinated. In this study cohort, thirteen patients who received extracorporeal membrane oxygenation and seven who died were unvaccinated. This report implies that two doses of the BNT162b2 mRNA vaccine reduce the risk of hospitalization due to COVID-19 infection by 94% among adolescents. Also, vaccination averted death or life support requirements for severely ill COVID-19 adolescents.
The result of this study is in line with the efficacy data from the BNT162b2 clinical trial involving adolescents (12 and 15 years of age) that reported vaccine efficacy of 100% against non-hospitalized COVID-19 illness.
Scientists stated that vaccine protection might vary in adolescents with underlying medical conditions. This group was excluded in clinical trials but was included while analyzing the vaccine's effectiveness in the real-world setting. Additionally, the efficacy of the vaccine may vary across different SARS-CoV-2 variants along with the interval since vaccination.
This study reported that Black and Hispanic populations are at a higher risk of COVID-19 infection compared to White children in the United States. In addition, the authors highlighted that the adolescent patients with underlying conditions and those from minority populations were underrepresented in the BNT162b2 clinical trial. However, this study showed that vaccination with BNT162b2 reduced the overall risk of hospitalization and ICU admission by 94% and 98%, respectively.
Conclusion
One of the strengths of this study is its longer follow-up duration (90 days) compared to the earlier BNT162b2 clinical trial (60 days). The current study has some limitations, which include insufficient sequencing, due to which the efficacy of the vaccine against specific SARS-CoV-2 variants could not be determined.
The Delta variant had been the dominant variant during the study period. Although the effectiveness of a single dose of vaccine was found to be high, the duration of protection from one dose has not been determined. The authors emphasized that more effort must be directed towards vaccinating adolescents, especially, those with underlying health conditions, as all the deaths occurred among unvaccinated patients.