Among the neurological damage is loss of myelin and increases in neuroinflammation that harm different types of brain cells. A lack of myelin in neurons threatens the health of current brain cells and impairs neural communication.
“The findings presented here illustrate striking similarities between neuropathophysiology after cancer therapy and after SARS-CoV-2 infection, and elucidate cellular deficits that may contribute to lasting neurological symptoms following even mild SARS-CoV-2 infection,” conclude the research team.
Study: Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. Image Credit: Design_Cells / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Experimental model set-up
The research team used a mouse model to simulate mild COVID-19 infection in the lungs only. Because severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection requires human ACE2 expression, the researchers inserted human ACE2 through a viral vector in the trachea and lungs. Two weeks after delivering human ACE2, a sample of SARS-CoV-2 was administered to mice through an intranasal route. The control group was given a viral vector without human ACE2 and also had a mock infection intranasally.
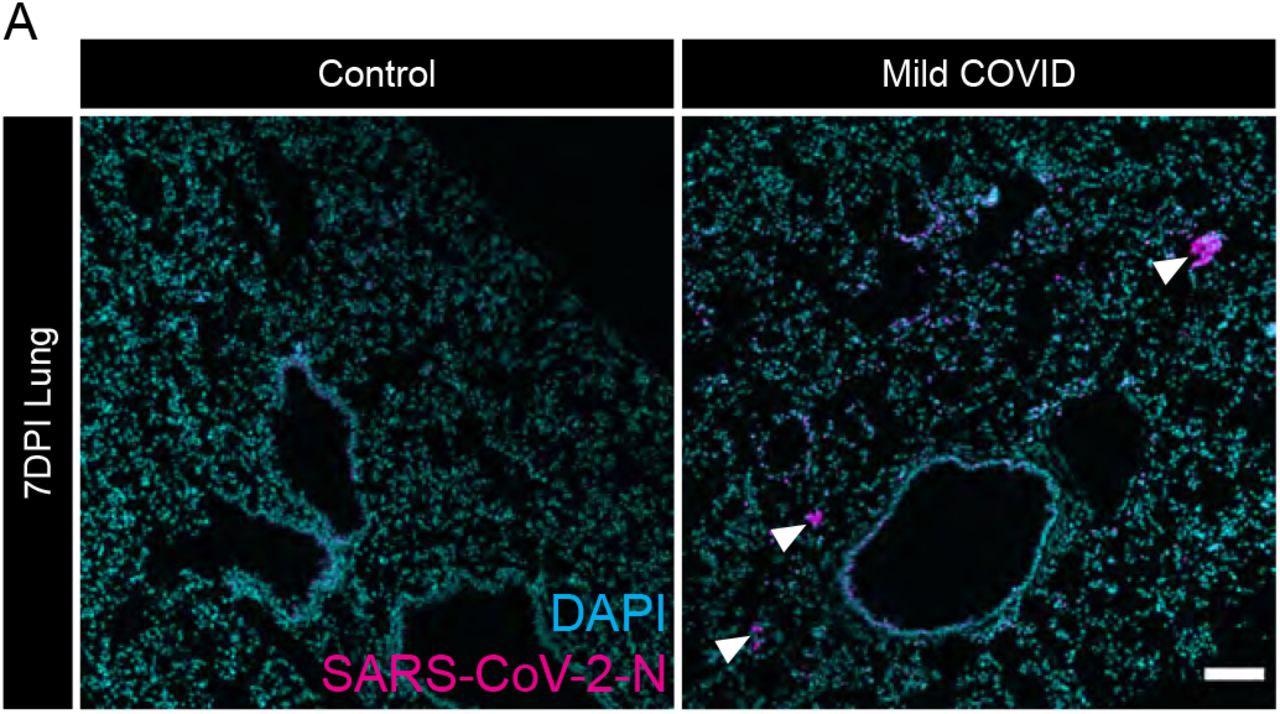
Evidence of SARS-CoV-2 infection in lung of mild respiratory COVID mouse model (A) Representative confocal micrographs of SARS-CoV-2 nucleocapsid protein (SARS- CoV-2-N, magenta; DAPI, cyan) in mouse lung 7-days post-infection. Arrowheads highlight SARS-CoV-2-N nucleocapsid protein immunostaining. Scale bar 100μm.
The infected mice showed no noticeable signs of weight loss or sickness. The researchers confirmed that SARS-CoV-2 was only in the lungs, not the brain.
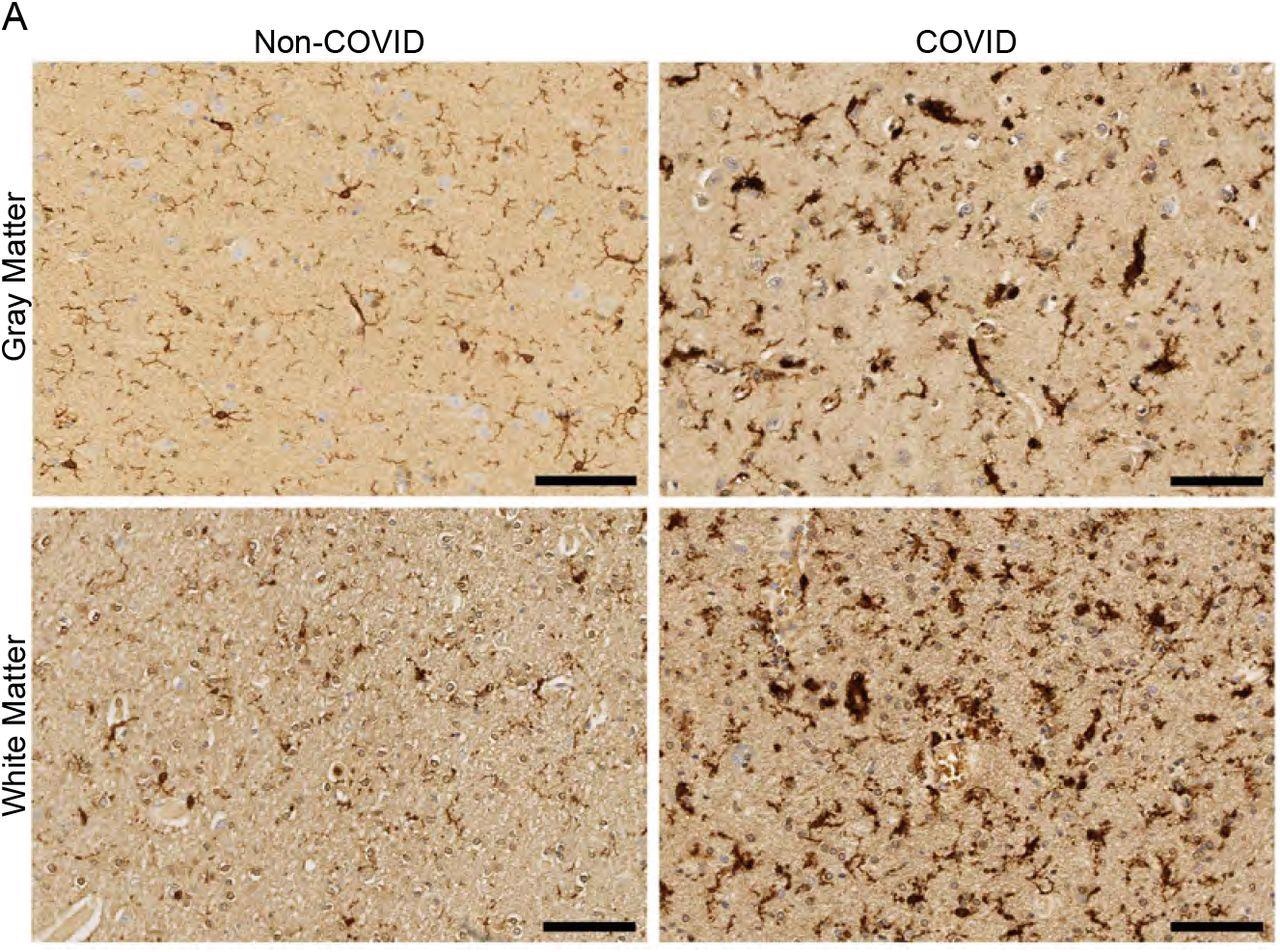
White matter-selective microglial reactivity in humans with SARS-CoV-2 infection (A) Representative micrographs of IBA1 immunostaining (brown) in the cerebral cortex (gray matter) or subcortical white matter of human subjects with or without COVID. Scale bars 100μm.
Respiratory COVID-19 infection changes cerebrospinal fluid levels
While mice with confirmed respiratory infection did not exhibit symptoms, the researchers discovered high cytokine levels in their cerebrospinal fluid (CSF) a week after infection. The team also found increased amounts of CSF cytokines and chemokines such as CXCL10, IL6, IFN-g, CCL7, CCL2, CCL11, and BAFF.
The elevated levels continued to show in CSF 7 weeks after. The elevated levels in certain chemokines and cytokines — CXCL10, CCL7, and CCL11 — also remained high for 7 weeks.
The findings suggest SARS-CoV-2 infection can cause changes to cytokines within the CSF even if there is no direct viral invasion in the brain.
Increased microglial activity in subcortical areas of the brain after infection
A week after infection, mice with mild COVID-19 infection showed increased microglial activity in the subcortical white matter. The changes persisted for 7 weeks. There were no changes to cortical gray matter.
The researchers also examined nine brains of patients who died from COVID-19 infection in 2020. Seven brains were male, and two were female. The ages ranged from 24 to 73. Only 1 patient was considered to have severe COVID-19 infection and required ICU admission. A control group was comprised of 5 brains from patients who died but were not infected with SARS-CoV-2.
The mouse models showed that mild or asymptomatic COVID-19 infection in humans caused increases in CD68+ microglial reactivity in subcortical white matter but not cortical gray matter.
Mouse model shows SARS-CoV-2 interferes with areas involved in cognition
When looking at the hippocampus of infected mice, results showed increased microglial reactivity in hippocampal white matter 7 days after infection, which persisted for 7 weeks.
Elevated levels of reactive microglia interfered with creating new neurons for the hippocampus, a critical site for learning and memory. As a result, there was a significant reduction in new neurons in the hippocampus a week after infection. Low neurogenesis was still evident after a 7-week follow-up.
The elevated levels of cytokines within CSF after mild infection is a sign of elevated IL6 levels in CSF a week after infection and increases in cytokine CCL11 after 7 weeks.
In long haulers (people with long COVID)’s plasma samples, increased CCL11 levels were associated with brain fog.
SARS-CoV-2 damages brain cells involved in making myelin
Next, the researchers looked at how mild infection affected oligodendrocytes in subcortical white matter. Oligodendrocytes are responsible for creating a protective coating called the myelin sheath to all neurons in the central nervous system. The myelin sheath is also essential in allowing rapid communication between neurons.
After 7 weeks, the team noticed an approximate 10% decrease in the number of oligodendrocyte precursor cells. Additionally, there were low levels of mature oligodendrocytes, with almost one-third gone a week following infection. Depleted amounts of mature oligodendrocytes continued for 7 weeks.
Given the lack of oligodendrocytes, there was also a substantial decrease in myelin. Reduced myelinated axons in subcortical white was observed a week after infection and persisted for 7 weeks.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Anthony Fernández-Castañeda et al. (2022). Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. medRxiv. doi: https://doi.org/10.1101/2022.01.07.475453, https://www.biorxiv.org/content/10.1101/2022.01.07.475453v1
- Peer reviewed and published scientific report.
Fernández-Castañeda, A., Lu, P., Geraghty, A. C., Song, E., Lee, M.-H., Wood, J., O’Dea, M. R., Dutton, S., Shamardani, K., Nwangwu, K., Mancusi, R., Yalçın, B., Taylor, K. R., Acosta-Alvarez, L., Malacon, K., Keough, M. B., Ni, L., Woo, P. J., Contreras-Esquivel, D., & Toland, A. M. S. (2022). Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell, 185(14), 2452-2468.e16. https://doi.org/10.1016/j.cell.2022.06.008. https://www.sciencedirect.com/science/article/pii/S0092867422007139.