The coronavirus disease-2019 (COVID-19) pandemic, caused by the rapid outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has massively affected the global healthcare system and economy.
The virus has been characterized to be highly transmissible and virulent. To date, it has infected around 330 million people and claimed more than 5.54 million lives worldwide. Among all age groups, older people and individuals with higher comorbidities are at a higher risk of SARS-CoV-2 infection.
 Study: Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. Image Credit: NIAID
Study: Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. Image Credit: NIAID
Importance of Animal Models
SARS-CoV-2 belongs to the family Coronaviridae. Other members of this family that have caused sporadic outbreaks are Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV).
In response to the COVID-19 pandemic, scientists developed several new vaccines and therapeutics and repurposed existing antiviral drugs to protect people from infection. It is, therefore, imperative to analyze the effectiveness of the newly developed vaccines, therapeutics, and repurposed drugs.
In order to study critical aspects of viral infection, replication, pathogenesis, and transmission, and more importantly, to detect vaccine candidates, animal models that support SARS-CoV-2 infection and recapitulate COVID-19 are urgently needed.
Even though multiple animal models have been proposed, including the Syrian golden hamster, the ferret, and nonhuman primates, none of these provide the tools for an in-depth analysis that mice do.
Although mice are the most popularly used animal model in laboratory research because of their small size, low maintenance cost, and fast reproduction time, they are not used in SARS-CoV-2 studies. This is because SARS-CoV-2 cannot use mouse orthologue of its human entry receptor angiotensin-converting enzyme 2 (hACE2) and, thereby, does not support the infection. However, some studies have revealed that SARS-CoV could infect mice, causing mild symptoms.
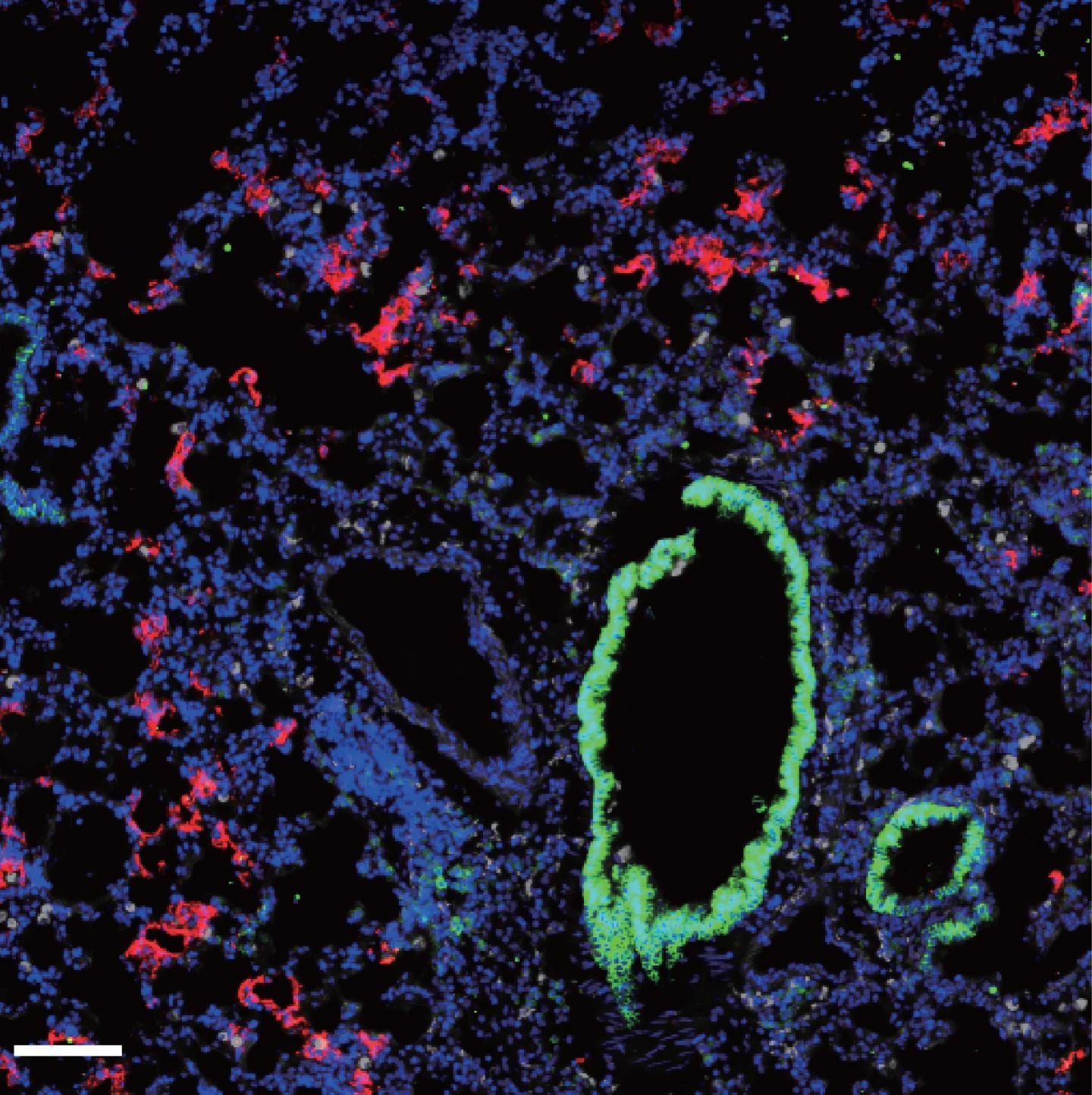 Fluorescence microscopy shows the presence of SARS-CoV-2 (red) within the lungs of mice expressing the human ACE2 protein. CREDIT © 2020 Israelow et al. Originally published in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20201241
Fluorescence microscopy shows the presence of SARS-CoV-2 (red) within the lungs of mice expressing the human ACE2 protein. CREDIT © 2020 Israelow et al. Originally published in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20201241
Coronavirus Infections and Interferons
Scientists have previously developed a mouse-adapted SARS-CoV model to understand how SARS-CoV infects humans. This study revealed the immune correlates of pathogenesis and protection. Importantly, it helped scientists discover that type I interferons (IFN) signaling was pathogenic in the context of SARS-CoV infection. However, the first mouse model of MERS-CoV infection showed that type I IFN signaling provided protection rather than pathogenic infection.
Previous studies have shed light on the different roles of type I IFN signaling, such as protecting from viral infections by developing adaptive immunity. However, these studies have also reported that many viral and bacterial infections and autoimmune diseases promote unregulated IFN signaling, which results in adverse effects or pathology.
A New Study
Recently, a study has shown the possibility of repurposing hACE2 transgenic mice, primarily raised to study SARS-CoV, to support SARS-CoV-2 infection and pathogenesis.
Although this mouse model provided a robust platform for studying SARS-CoV-2 infection, it had certain limitations, including inadequate availability of transgenic mice and limited to a single genetic background.
The study published in the Journal of Experimental Medicine has focused on developing a mouse model that supports SARS-CoV-2 infection and pathogenesis. This model is based on adeno-associated virus (AAV)–mediated expression of hACE2. One of the advantages of using AAV is that it has significantly less immunogenicity, which is essential for studying immune responses during viral infections. In this mouse model, mice of diverse genetic backgrounds were infected with authentic COVID-19 patient-derived SARS-CoV-2 virus.
Scientists revealed that the newly developed mice model helped understand SARS-CoV-2 replication, antibody production and also displayed pathological manifestations analogous to COVID-19 patients. For instance, the model supported inflammatory pulmonary infiltrates characteristic of COVID-19 in humans.
This study observed that viral clearance was not significantly improved by the lack of IFN signaling, implying insufficient endogenous IFN signaling for clearance of SARS-CoV-2 infection. However, researchers confirmed that IFN regulates a pro-inflammatory immune response, characterized by the recruitment of pro-inflammatory monocytes and macrophages, and activates CD4+, CD8+, and natural killer cells. This finding indicates the role of IFN in immunopathology in COVID-19 patients.
The infected mice also possessed gene signatures of acute ISG response, which is closely associated with type I IFN response. Additionally, IgG and neutralizing antibodies post SARS-CoV-2 infection were also reported. None of the studied mice showed infection-related mortality, which might be owing to the immune status of the challenged mice that were 6 to 12 weeks old.
Conclusion
The AAV-hACE2 mouse model helped perform in-depth analysis following SARS-CoV-2 infection. The new study also reported that although type I IFNs marginally control SARS-CoV-2 replication, they are responsible for significant pathological outcomes.
In the future, more studies involving aged, immunocompromised, and/or obese mice are required that would help understand why elderly or individuals with comorbidities are more vulnerable to severe SARS-CoV-2 infection.
The authors stated that this model provides a much-needed platform that supports rapid testing of prophylactic and therapeutic strategies to contain the COVID-19 pandemic.