The findings from a recent study conducted in the United States suggest that combining saliva with a practical pooling protocol may open the door for easier surveillance testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), particularly in resource-limited settings. The research paper is currently available on the medRxiv* preprint server while it undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The pandemic of coronavirus disease 2019 (COVID-19) has shown that widespread surveillance of asymptomatic individuals should be regarded as one of the pivotal steps in the control of SARS-CoV-2 spread.
One of the resource-saving approaches is the pooling of samples prior to testing to increase testing capacity, most notably for surveillance in a population with relatively low infection rates, such as schools or for travelers.
Likewise, testing specific community members may serve as a proxy to the broader community and can potentially identify more significant outbreaks through family members and their associated activities.
Since saliva is easily collected from a large number of individuals, pooling strategies can be viewed as a natural extension to surveillance programs. And while pooling saliva does impact assay sensitivity and potentially decreases virus detection, thus far, the research has shown that the actual impact appears to be minimal.
A research group from the Department of Epidemiology of Microbial Diseases, Yale School of Public Health and Flambeau Diagnostics in Madison, US, recently developed the SalivaDirect RT-qPCR assay to increase SARS-CoV-2 testing access with a low-cost, streamlined protocol that uses self-collected saliva specimens.
A detailed workflow evaluation
In their methodological approach, this research group explored different extraction-free pooled saliva testing workflows before testing it with the SalivaDirect assay in order to expand the single sample testing protocol.
Upon identifying the optimal pool size, they have performed an initial workflow evaluation by using five different pooled samples – each consisting of a single SARS-CoV-2-positive saliva sample in combination with four SARS-CoV-2-negative saliva samples (with a 1:5 dilution).
Furthermore, to appraise the real-world potential loss of sensitivity on clinical samples with pooling, they have estimated the average change in cycle threshold (Ct) value for each pooled testing workflow by using the results from the 25-sample workflow confirmation study.
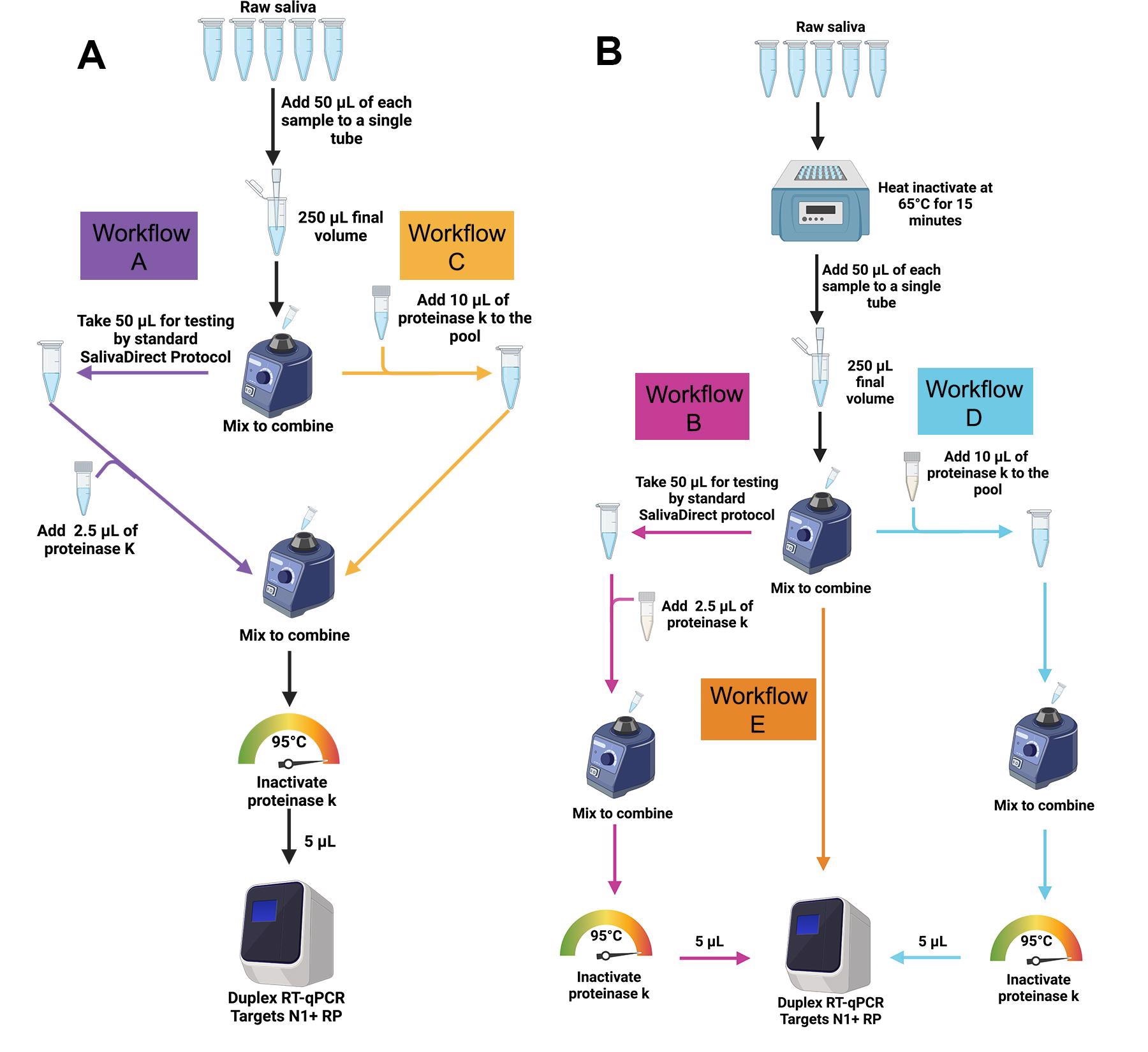 The SalivaDirectTM pooled testing workflows were evaluated in the study. (A) For workflows A (purple) and C (gold), 50 μl of SARS-CoV-2-positive saliva and 200 μL total volume of four SARS-CoV-2-negative saliva samples (50 μl each) were pooled and vigorously vortexed to mix. For workflow A, 50 μl of the pooled sample was tested following the standard SalivaDirect protocol. For workflow C the remaining sample was treated with 10 μl of proteinase K then heat-inactivated before testing directly without further treatment in the SalivaDirectTM RT-qPCR assay. (B) For workflows B, D and E, individual, non-pooled samples were incubated at 65ºC for 15 minutes before combining 50 μl of each sample into pools of 5 pre-treated samples. For workflow B (pink), 50 μl of the pool of pretreated samples was tested through the standard SalivaDirect protocol. For workflow E (orange), 10 μl of each of these pre-treated pools was removed and tested with the SalivaDirectTM RT-qPCR assay without proteinase K treatment. Finally, for workflow D (blue), 10 μl of proteinase K was added to the remaining volume of the pre297 treated pool then heat-inactivated before testing in the SalivaDirectTM RT-qPCR assay.
The SalivaDirectTM pooled testing workflows were evaluated in the study. (A) For workflows A (purple) and C (gold), 50 μl of SARS-CoV-2-positive saliva and 200 μL total volume of four SARS-CoV-2-negative saliva samples (50 μl each) were pooled and vigorously vortexed to mix. For workflow A, 50 μl of the pooled sample was tested following the standard SalivaDirect protocol. For workflow C the remaining sample was treated with 10 μl of proteinase K then heat-inactivated before testing directly without further treatment in the SalivaDirectTM RT-qPCR assay. (B) For workflows B, D and E, individual, non-pooled samples were incubated at 65ºC for 15 minutes before combining 50 μl of each sample into pools of 5 pre-treated samples. For workflow B (pink), 50 μl of the pool of pretreated samples was tested through the standard SalivaDirect protocol. For workflow E (orange), 10 μl of each of these pre-treated pools was removed and tested with the SalivaDirectTM RT-qPCR assay without proteinase K treatment. Finally, for workflow D (blue), 10 μl of proteinase K was added to the remaining volume of the pre297 treated pool then heat-inactivated before testing in the SalivaDirectTM RT-qPCR assay.
Minimal impact on assay sensitivity
In this study, a pool size of five (with or without heat inactivation for 15 minutes prior to testing) resulted in a positive agreement of 98% and 89%, respectively. Furthermore, an increased Ct value shift of 1.37 and 1.99 has been observed in comparison to individual testing of the positive clinical saliva specimens.
When this shift in Ct value has been applied to 316 individuals, consecutively collected SARS-CoV-2 positive saliva specimen results reported from six laboratories that used the original SalivaDirect assay, 100% of the samples would have been detected had they been tested in the 1:5 pool strategy.
The approach put forth in this study shows minimal impact on assay sensitivity with five samples per pool, and are basically straightforward extensions of a simple SARS-CoV-2 testing method that can be quickly conducted manually, without the need for any additional investment.
Towards facilitated SARS-CoV-2 surveillance
In a nutshell, the rationale behind this study and the findings suggest that combining saliva with a practical pooling protocol may enable facilitated SARS-CoV-2 surveillance testing, which is especially pertinent for resource-limited settings.
“Such pooled testing has the potential to significantly reduce the overall number of tests and associated costs”, say study authors in this medRxiv paper. “This would in turn operationally permit an increased frequency of testing, meaning an increased likelihood of detecting individuals earlier in their infection”, they add.
Basically, this approach is a way to implement broader screening in schools and workplaces for SARS-CoV-2 testing, laying the foundation for the optimized management of any future upper respiratory infection mediated pandemics.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Allicock, O.M., Yolda-Carr, D., Todd, J.A. & Wyllie A.L. (2022). Pooled RNA: extraction free testing of saliva for SARS-CoV-2 detection. medRxiv. https://doi.org/10.1101/2022.01.16.22269390, https://www.medrxiv.org/content/10.1101/2022.01.16.22269390v1
- Peer reviewed and published scientific report.
Allicock, Orchid M., Devyn Yolda-Carr, John A. Todd, and Anne L. Wyllie. 2023. “Pooled RNA-Extraction-Free Testing of Saliva for the Detection of SARS-CoV-2.” Scientific Reports 13 (1): 7426. https://doi.org/10.1038/s41598-023-34662-2. https://www.nature.com/articles/s41598-023-34662-2.