Recent studies have reported that some vaccines have NSE and can potentially provide protection against different pathogens. Although many economic evaluations analyze the pathogen-specific effects of vaccines, only two economic evaluations of NSE were available. This study attempts to examine economic assessments of the NSE of OPV against coronavirus disease 2019 (COVID-19) and under-five mortality.
Study: One vaccine to counter many diseases? Modelling the economics of oral polio vaccine against child mortality and COVID-19. Image Credit: frank60 / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study
The researchers evaluated two settings: 1. reducing child mortality in Guinea-Bissau, a high-mortality setting where many previous studies, including randomized controlled trials, found that OPV reduced child morbidity and mortality by 10-36% and 2. prevention of COVID-19 in India, a lower-middle-income setting that faces delays in COVID-19 vaccine availability due to delays in vaccine development, production, and supply chain.
Concerning reducing child mortality, three annual campaigns were modeled in which all eligible children receive one bivalent OPV dose every year for three years compared to a hypothetical cohort who did not receive the vaccine. The researchers analyzed a birth cohort of one million children in Guinea-Bissau with a death rate of was 78 per 1,000 live births among children under five years of age in 2019. It was assumed that all newborns received a standard OPV dose at birth and three OPV doses at 6, 10, and 14 weeks of age, along with other routine vaccination.
In terms of COVID-19 prevention, the team developed a susceptible-exposed-infectious recovered model to measure the population benefits of two different scenarios where OPV would be co-administered with COVID-19 vaccines. In addition, they adapted the model to reflect the COVID-19 transmission dynamics more accurately.
The researchers first expanded the “infectious” compartment into symptomatic, asymptomatic, hospitalized, and intensive care to account for varying levels of disease severity. Then they created two more parallel sets of compartments for vaccinated individuals to reflect the reduction in transmission and COVID-19 severity after vaccination. To account for uncertainty and heterogeneity, they modeled benefit-cost ratios and incremental cost-effectiveness for intervention effectiveness estimate ranges.
Results
For child mortality, the results show that headline cost-effectiveness was $650 per child death prevented. The benefit-cost ratio was 110 with a value per statistical life (VSL) of $71,000. Without the intervention, Guinea-Bissau’s age-specific death rates would be 50,000 in children aged between 0 and 1 year, 6,650 deaths between ages 1 and 2 years, and 6,600 deaths between 2 and 3 years. The results showed that, with the OPV intervention, the total number of deaths averted by the campaigns is 5,990, a 9.5% death reduction from the numbers without the campaign.
The authors expect higher averted deaths per 1000 campaign doses and a lower cost-effectiveness ratio with a higher mortality rate under five years and intervention effectiveness. The cost-effectiveness or cost-benefit may decline with a lower mortality rate or intervention effectiveness.
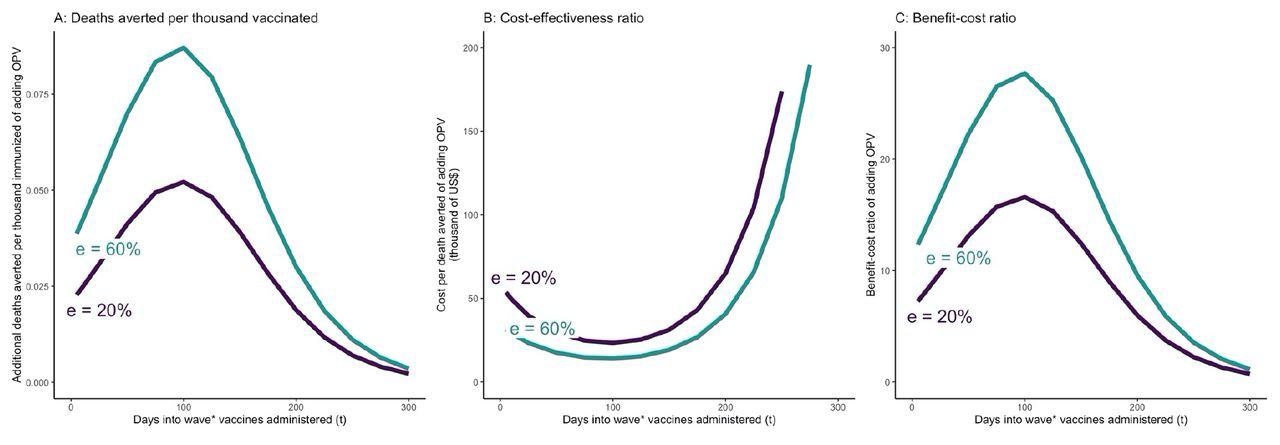
OPV and COVID-19 vaccine simultaneously administered t days after the beginning of epidemic wave – incremental mortality impact, cost-effectiveness, and benefit-cost of adding OPV A: Incremental deaths averted per 1000 individuals of adding OPV to COVID-19 vaccine only schedule B: Incremental cost-effectiveness ratio per averted death of adding OPV to COVID-19 vaccine only schedule C: Incremental benefit-cost ratio of adding OPV to COVID-19 vaccine only schedule The results to reflect the scenario in which vaccine coverage for both vaccines are set at 30%. OPV is administered simultaneously with the COVID-19 vaccine on day t. The COVID-19 vaccine is assumed to be 74% effective against infections and reducing infectivity, 95% effective against severity, and requires 28 days from administration until it becomes fully effective. e = effectiveness of OPV vaccine against COVID-19.
For COVID-19, assuming an OPV effectiveness of 20%, incremental cost was $23,000-65,000 per 58 deaths averted if OPV was administered along with a COVID-19 vaccine within 200 days of an epidemic wave. At a 30% vaccine coverage rate, co-administering OPV along with COVID-19 vaccine would lead to a 13-35% reduction in COVID-19 infections and deaths if the vaccines are administered prior to day 100, and a 2-13% reduction if the vaccines are administered between day 100 and 200, compared to the scenario where only COVID-19 vaccines are administered.
In case of delay in the availability of COVID-19 vaccines, the cost per averted death would decline to $2600-6100. The estimated benefit-to-cost ratios were consistently high, though they varied considerably.
Conclusion
The results of the economic evaluation performed in this study highlight the potential of OPV to reduce child mortality in high mortality settings and its economically attractive role in COVID-19 prevention. The findings are in line with previous and ongoing studies that have shown the high effectiveness of LAVs in activating innate immune responses and reducing disease burden.
The current findings show that LAVs such as OPV can be cost-effective as well as cost-beneficial interventions that can reduce child mortality. Although this evaluation shows the attractive potential of LAVs against COVID-19, this needs to be further investigated in trials.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
One vaccine to counter many diseases? Modelling the economics of oral polio vaccine against child mortality and COVID-19. Angela Y. Chang, Peter Aaby, Michael S. Avidan, Christine S. Benn, Stefano M. Bertozzi, Lawrence Blatt, Konstantin Chumakov, Shabaana A. Khader, Shyam Kottilil, Madhav Nekkar, Mihai G. Netea, Annie Sparrow, Dean T. Jamison. medRxiv 2022.01.19.22269560, doi: https://doi.org/10.1101/2022.01.19.22269560, https://www.medrxiv.org/content/10.1101/2022.01.19.22269560v1
- Peer reviewed and published scientific report.
Chang, Angela Y., Peter Aaby, Michael S. Avidan, Christine S. Benn, Stefano M. Bertozzi, Lawrence Blatt, Konstantin Chumakov, et al. 2022. “One Vaccine to Counter Many Diseases? Modeling the Economics of Oral Polio Vaccine against Child Mortality and COVID-19.” Frontiers in Public Health 10 (October). https://doi.org/10.3389/fpubh.2022.967920. https://www.frontiersin.org/articles/10.3389/fpubh.2022.967920.