
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Several studies have demonstrated an attenuated immune response after vaccination in organ transplant recipients and immunosuppressed individuals. To evade this, many countries, including the United Kingdom, have sanctioned an additional coronavirus disease 2019 (COVID-19) vaccine doses for this subset of their population.
Preliminary reports have shown that heterologous vaccination regimens provide equivalent immunogenicity in both transplant recipients and the general population. While most countries employing this vaccination strategy almost exclusively used messenger ribonucleic acid (mRNA)-based vaccines, such as the Pfizer-BioNTech BNT162b2 vaccine, the U.K. rolled out a heterologous vaccination approach from September 2021. To this end, the U.K. solely used the BNT162b2 vaccine as a third dose in transplant recipients immunized with the AstraZeneca ChAdOx1 or BNT162b as their first two doses.
About the study
The present study included 700 kidney transplant recipients under treatment at the Imperial College Renal and Transplant Centre in London. The researchers used a non-quantitative Abbott Architect SARSCoV-2 immunoglobulin G (IgG) two-step chemiluminescent immunoassay (CMIA) for detection of anti-nucleocapsid (N) protein, interpreted as positive or negative with a threshold index value of 1.4. Using the Abbott Architect SARS-CoV-2 IgG Quant II CMIA, the researchers detected spike (S) protein antibody titers (anti-S) with a threshold value for positivity of 7.1 BAU/ml.
All 700 subjects had received three primary doses of a SARS-CoV-2 vaccine, wherein 52.3% had received BNT162b2 as the first two doses and 47.7% had received ChAdOx1. However, all study participants received the BNT162b2 vaccine as their third dose.
All study participants were vaccinated after their transplantation surgery and underwent serological testing after the second vaccination at a median time of 34 days. In total, 19.9% of subjects had evidence of prior SARS-CoV-2 infection, of which 75 and 64 patients had received BNT162b2 and ChAdOx1 as their first two doses, respectively.
Study findings
In infection naïve subjects, 46.9% had no detectable anti-S titers following two doses of vaccine (V2), with 23.9% remaining seronegative at a median time of 33 days post-third vaccine (V3). The proportion of seronegative participants among those who received three doses of the BNT162b2 vaccine remained significantly lower than those who had received the first two doses of the ChAdOx1 vaccine.
After V3, median anti-S concentrations of patients who had received BNT162b2 and ChAdOx1 vaccines were 612 BAU/ml and 122 BAU/ml, respectively. Following V2 as compared with V3, anti-S concentrations significantly increased from 45 BAU/ml to 612 BAU/ml and 7.1 to 122 BAU/ml in recipients of BNT162b2 and ChAdOx1 vaccines, respectively.
In all 139 subjects with prior infection, 2.9% remained seronegative following the third dose, whereas one was anti-N positive. The median anti-S concentrations in subjects with previous infection post-V3 were 4,064 BAU/ml.
There was no difference in anti-S concentrations post-V3 in participants who had received BNT162b2 or ChAdOx1 as their first two doses, with concentrations of 5,680 BAU/ml compared with 3,941 BAU/ml, respectively.
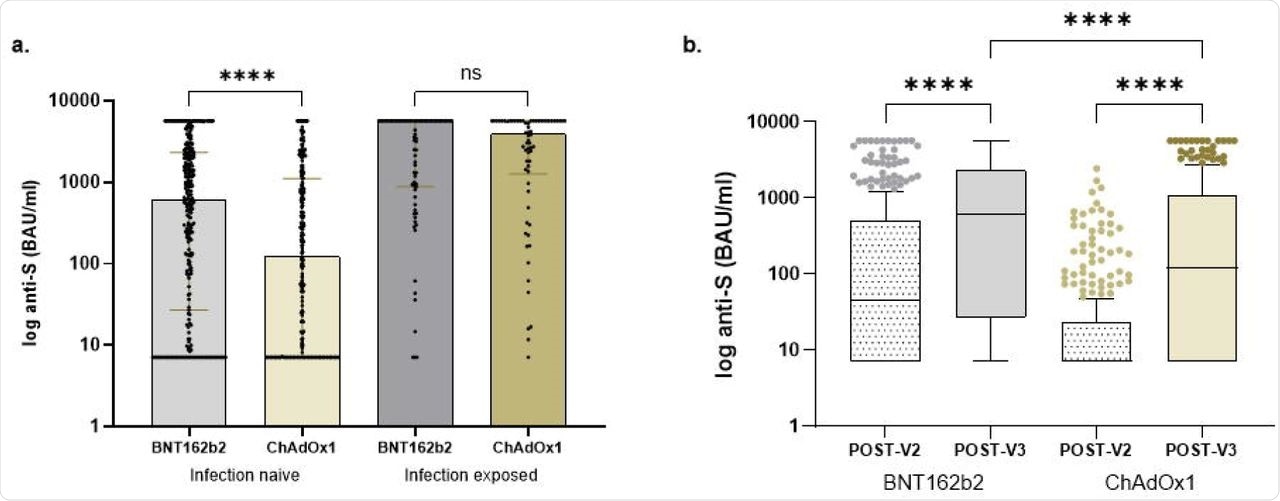
Comparison of anti-S concentrations in transplant recipients.
Upon evaluating T-cell responses in 30 infection-naïve subjects at a median time of 35 days after V3, 60% of participants had undetectable T-cell responses. This indicated a positive correlation between quantitative serological and cellular responses.
In transplant patients who received the first two doses of BNT162b2 or ChAdOx1, ten and eight patients had undetectable T-cell responses, respectively, thus indicating that T-cell response variations between patients were neither qualitative nor quantitative. A trend towards an inverse correlation between increasing age and the T-cell response was also observed.
Conclusions
To summarize, the results of the current study showed that serological responses in transplant patients with no prior infection were effective when immunized with a homologous three-dose vaccination regimen of the BNT162b2 vaccine. The measured cellular responses also appeared the same in both groups.
In general, T-cell immunity correlates with B-cell responses; however, transplant patients on T-cell-directed immunosuppressive agents have predominant cellular immunity. Therefore, the authors suggest that future studies consider evaluating T-cell responses in transplant patients.
These findings, combined with real-world data on the protection conferred upon transplant recipients by vaccination and the impact of the different types of COVID-19 vaccines, are crucial to the strategic planning to protect transplant recipients.
Further, such approaches will need customization for each patient, SARS-CoV-2 variant, and infection rates. Therefore, for patients who are non-responsive to three doses of vaccination, alternative immune protection will have to be offered. If these individuals are still subjected to repeated vaccine dosing, the merits of heterologous vaccinations will need to be investigated.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Thomson, T., Prendecki, M., Gleeson, S., et al. (2022). Comparative immunogenicity of heterologous versus homologous 3rd SARS-CoV-2 vaccine doses in kidney transplant recipients. medRxiv. doi:10.1101/2022.01.25.22269778. https://www.medrxiv.org/content/10.1101/2022.01.25.22269778v1.
- Peer reviewed and published scientific report.
Thomson, Tina, Maria Prendecki, Sarah Gleeson, Paul Martin, Katrina Spensley, Rute Cardoso De Aguiar, Bynvant Sandhu, et al. 2022. “Immune Responses Following 3rd and 4th Doses of Heterologous and Homologous COVID-19 Vaccines in Kidney Transplant Recipients.” EClinicalMedicine 53 (November): 101642. https://doi.org/10.1016/j.eclinm.2022.101642. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00372-8/fulltext.