Coronaviruses (CoVs) are a varied group of single-stranded positive-sense ribonucleic acid (RNA) viruses that can infect humans and other mammals. Several different CoVs have appeared in human populations in the last 20 years.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
Seasonally circulating human CoVs including HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 have long been known to cause minor respiratory tract infections. The severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV-2, on the other hand, are highly pathogenic.
SARS-CoV-2 was initially identified as the virus responsible for the coronavirus disease 2019 (COVID-19) outbreak that began in Wuhan, China in December 2019. COVID-19 was declared a pandemic by the World Health Organization in March 2020, with more than 375 million confirmed cases and almost 5.7 million deaths documented as of January 31, 2022. Sequence alterations, particularly in the spike protein, and the subsequent introduction of novel variants have been discovered by large-scale whole-genome sequencing (WGS) of the SARS-CoV-2.
The Omicron variant, which is the most recently identified variant of SARS-CoV-2, is associated with exceptional proliferation. As a result, governments around the world are adopting fast antigen tests to quickly identify infected individuals and reduce virus transmission.
To date, only a few studies have assessed the accuracy of antigen tests at detecting SARS-CoV-2 variants, particularly Omicron. In the current study published on the medRxiv* preprint server, researchers determined the analytical sensitivity of a CE-marked fast antigen with the Omicron, Delta, Alpha, and Gamma variants. Their findings show that the antigen test is successful at detecting SARS-CoV-2 variants, including the currently dominant Omicron variant, despite minor differences in sensitivity.
About the study
In the current study, the authors used clinical isolates of Omicron, Delta, Alpha, and Gamma to determine the analytical sensitivity of a fast antigen test with SARS-CoV-2 variants. Each variant was diluted to 100,000 PFU/mL test samples, then serially diluted ten times to get 10,000, 1,000, 100, 10, and 1 PFU/mL test samples.
The detection limits were determined by applying 100 liters of test samples to each fast antigen test. Before the results were visually graded and photos were collected, the antigen tests reacted for a total of 15 minutes.
The Delta variant had the greatest limit of detection at 1,000 PFU/mL, followed by the Omicron variant at 100 PFU/mL, according to the fast antigen test. At 10 PU/mL, the fast antigen test exhibited the lowest detection limits against the Alpha and Gamma variants.
When using the 1 PFU/mL test samples from the Alpha and Gamma variants, as well as the kit buffer alone, the fast antigen test came back negative. The visual scoring results were backed up by image analysis of test signal intensities.
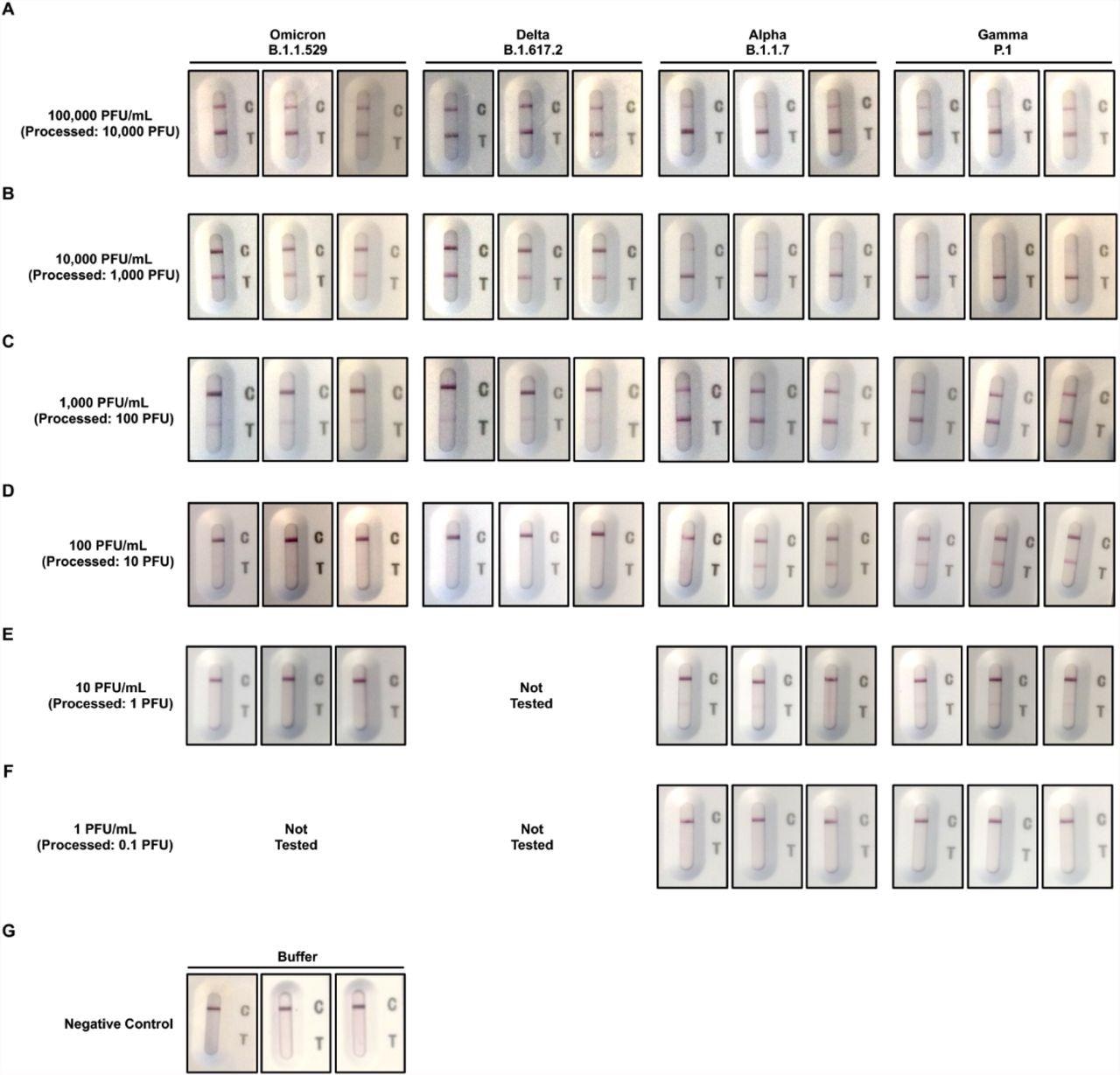
Images of rapid antigen tests for different concentrations of Omicron, Delta, Alpha, and Gamma variants recorded after 15 minutes. A) 100,000 PFU/mL. B) 10,000 PFU/mL. C) 1,000 PFU/mL. D) 100 PFU/mL. E) 10 PFU/mL. F) 1 PFU/mL. G) Kit buffer. All tests were carried out in triplicates.
Implications
Since the SARS-CoV-2 nucleocapsid (N) protein is one of the most commonly produced structural proteins, it is an excellent target for viral detection in infected patients. As a result, the majority of antigen assays have been established for SARS-CoV-2 N; however, changes in this protein can affect virus detection.
R230K and G204R mutations in N have been found in over 85% of Omicron, Alpha, and Gamma sequences. Those mutations, on the other hand, were found in less than 0.1% of Delta sequences. These findings may explain why the Delta fast antigen test has a lower analytical sensitivity than the other variants.
Another possibility is that the viral stocks utilized in the current study contained non-infectious particles, resulting in varying levels of N that could affect the test results' signal intensity. More research is needed to determine which mutations in the SARS-CoV-2 variants’ N protein have a negative impact on the detection limits of fast antigen tests.
Furthermore, investigations evaluating the performance of fast antigen tests utilizing nasal or oropharyngeal swab specimens from reverse-transcriptase polymerase chain reaction (RT-PCR) confirmed COVID-19 patients are needed. Taken together, the antigen test utilized in this study is successful at detecting SARS-CoV-2 variants, despite minor changes in analytical sensitivity.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.