Immunization is considered the most important and efficient strategy for preventing severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). However, the immune responses dwindle over time, posing threats of lower protection against novel virus variants.
Immunocompromised patients and those undergoing immunosuppressive therapy, such as kidney transplant recipients (KTRs), demonstrate lower peak antibody values and rapid waning of antibody levels than the general population. Thus, intensified vaccination schedules with early booster doses have been recommended in this population.
Still, the seroconversion rates range between 25-50%, which confers inadequate protection. The use of immunosuppressive agents like – mycophenolate mofetil (MMF) and belatacept present similar drawbacks among KTR patients—with respect to coronavirus disease 2019 (COVID-19) vaccination.
 Study: Immune response to third SARS-CoV-2 vaccination in seronegative kidney transplant recipients: possible improvement by mycophenolate mofetil reduction. Image Credit: NIAID
Study: Immune response to third SARS-CoV-2 vaccination in seronegative kidney transplant recipients: possible improvement by mycophenolate mofetil reduction. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study
A new study posted to the medRxiv* preprint server aimed to determine the durability of immune responses to COVID-19 vaccines by measuring the kinetics of antibody waning among seropositive KTRs. Here, factors influencing the immune response to the third COVID-19 vaccination dose were also assessed in seronegative KTRs. Additionally, the effect of temporary reduction of MMF in improving the immune response to the third COVID-19 vaccination dose was analyzed in a small group of KTRs.
In this prospective, multicenter, observational study, 245 KTRs who received two doses of either BNT162b2 (BioNTech/Pfizer) or mRNA-1273 (Moderna) were included. The participants were above 18 years of age and without a history of COVID-19 infection, on stable immunosuppressive therapy, showed no signs of acute graft rejection, and did not have a history of graft rejection within the last six months.
Findings
Here, antibody levels six months after the first vaccination dose were measured in the 60 participants who were seropositive after the second dose. It was noted that after three and six months, the antibody levels decreased by 45% and 73%, respectively, in this cohort. Due to this waning, nearly 30% KTRs became seronegative.
However, the neutralizing capacity against the Delta variant was significantly lower. Primary factors associated with the transition to a seronegative status over the study period were – initially low antibody levels, older age and impaired renal graft function. Of note, immunosuppression with the use of MMF did not correlate with seroconversion.
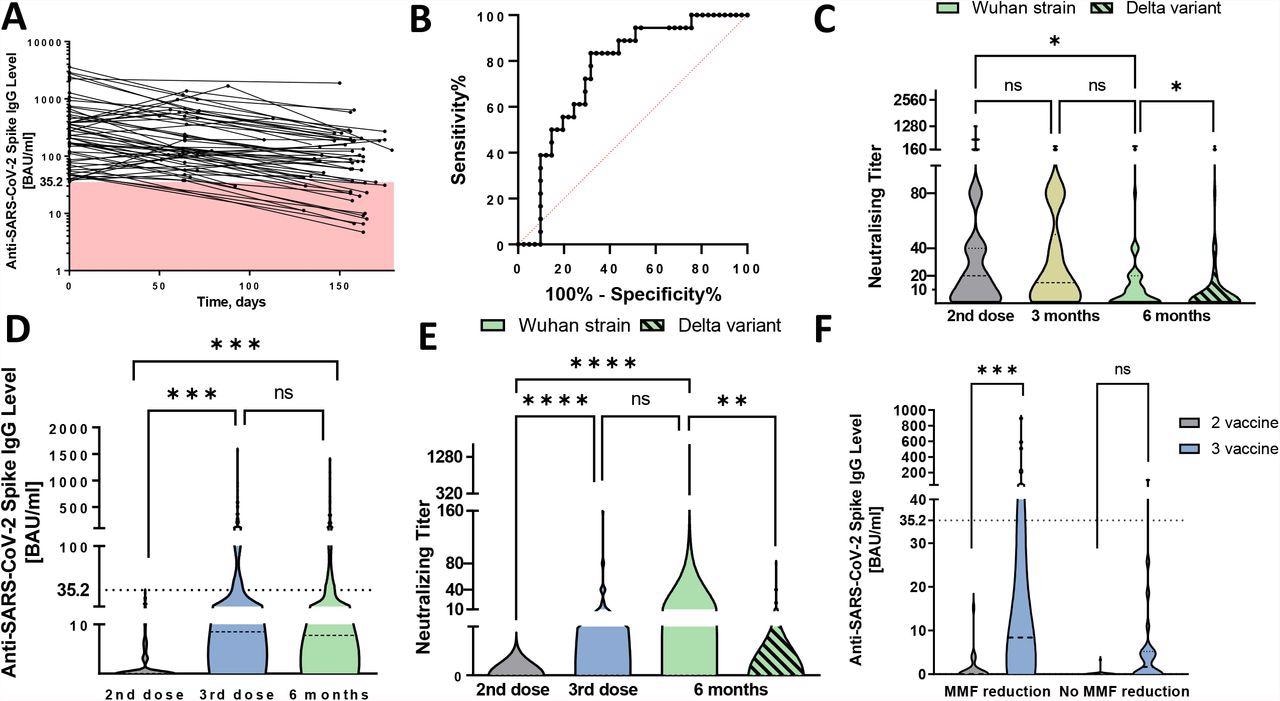
Antibody waning and humoral response to third SARS-CoV-2 vaccination among kidney transplant recipients. A Waning of anti-SARS-CoV-2 spike subunit S1 antibodies among 60 seropositive kidney transplant recipients (KTRs) after the first two vaccination over the follow-up period. The red-colored space highlights antibody levels lower than the cut-off for seropositivity (<35.2 BAU/ml). B Receiver operating characteristics (ROC) curve for antibody level with respect to serological patient status at six-month visit with AUC being 0.763 (95% CI 0.639–0.887), p=0.002. C Comparison of neutralizing antibodies among seropositive KTRs over the follow-up period. At the six months follow-up visit, serum was tested for neutralizing antibody capacity against the Wuhan strains and delta virus variant. D Comparison of antibody levels among KTRs after the third vaccination and at six months follow-up visit. Dashed line was set at 35.2 BAU/ml to outline seropositive patients. E Comparison of neutralizing titer among KTRs after the third vaccination and at six months follow-up visit. At the six months follow-up visit, patients’ serum was tested for neutralizing antibody capacity against the Wuhan strain and delta virus variant. F Comparison of antibody level between patients with and without MMF dose reduction. The graph represents immune response among 24 matched pairs using propensity score with respect to initial antibody concentration, renal graft function, time after transplantation, and MMF trough level prior to the dose reduction. Differences between different follow-up visits were analyzed using Kruskal-Wallis test. Differences in neutralizing capacity against Wuhan strain and delta variant were assessed using Wilcoxon signed-rank test. **** represent p value < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05.
Overall, 174 seronegative KTRs received the third COVID-19 vaccination dose. Based on the antibody levels detected 22±7 days post-vaccination, KTRs were divided into two groups – seronegative patients with antibody levels below 35.2 BAU/ml and seropositive patients with antibody level ≥35.2 BAU/ml. It was found that seroconversion after the third vaccination dose depended upon the antibody levels after the second vaccination dose and just after transplantation.
Higher antibody responses were seen in patients who had better renal graft function and lower trough MMF serum levels. In univariate regression analysis, a daily MMF dose ≤1g rendered 2.5 times higher chances for developing a clinically relevant quantity of antibodies.
In the 24 KTRs who were advised to reduce the daily MMF dose for three consecutive weeks--until one week after the third vaccination dose—a significant decline in MMF serum concentrations was observed. A significantly better immune response to the third COVID-19 vaccination dose was documented in this cohort, compared to matched controls.
In fact, after the reductions, 29.2% of patients achieved antibody levels above the threshold of a seropositive state. While among the matched controls, only 4.2% crossed this mark. Furthermore, in the MMF reduction group, these seropositive KTRs had lower MMF trough levels than the 17 seronegative KTRs. Meanwhile, for the 23 seronegative KTRs without MMF reduction, the MMF trough levels were also significantly higher than those for the MMF reduction group.
In this study, 30% KTRs transited to a seronegative state within six months from the first vaccination dose. The initial antibody level was the most crucial factor in predicting the decline below the seropositive cut-off.
The antibody waning trend in KTRs was identical to those observed in the general population. However, since the baseline antibody levels in KTRs are lower, the waning could be swifter. Therefore, booster vaccinations should be recommended after shorter intervals in this population.
Furthermore, KTRs exhibit neutralizing antibody levels decline more rapidly in KTRs—which also depends on the virus variant—for example, a lower amount of neutralizing antibodies against the Delta variant. Moreover, the lower immune response to the first two COVID-19 vaccination doses in KTRs was found to be related to immunosuppressive drug use, especially MMF.
With the third vaccination dose, an increase in trough MMF concentrations by 1μg/ml was associated with a nearly 60% lower seroconversion. Furthermore, below-threshold antibodies prior to the third vaccination dose were also associated with a better humoral immune response. Besides, the probability of attaining antibody levels after the third dose also increased with improved renal graft function and longer duration after post-transplantation.
The results indicated that MMF reduction could be achieved in minimal amounts for better immune response. However, low MMF concentrations may heighten the risk for graft rejection. Hence, close monitoring is necessary.
The findings revealed that antibody kinetics in KTRs is similar to the general population. However, these patients develop lower antibody levels – which confer shorter humoral protection. A moderate, temporary MMF dose reduction may be adopted for improving vaccination success in KTRs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kantauskaite, M., Müller, L., Hillebrandt, J., et al. (2022), “Immune response to third SARS-CoV-2 vaccination in seronegative kidney transplant recipients: possible improvement by mycophenolate mofetil reduction”, medRxiv preprint, 10.1101/2022.01.18.22269420, https://www.medrxiv.org/content/10.1101/2022.01.18.22269420v1
- Peer reviewed and published scientific report.
Kantauskaite, Marta, Lisa Müller, Jonas Hillebrandt, Joshua Lamberti, Svenja Fischer, Thilo Kolb, Katrin Ivens, et al. 2022. “Immune Response to Third SARS‐CoV‐2 Vaccination in Seronegative Kidney Transplant Recipients: Possible Improvement by Mycophenolate Mofetil Reduction.” Clinical Transplantation 36 (11). https://doi.org/10.1111/ctr.14790. https://onlinelibrary.wiley.com/doi/10.1111/ctr.14790.