Individuals with low immunoglobulin levels and reduced antibody responses to pathogens and vaccines are associated with common variable immune deficiency (CVID) and other primary antibody deficiency syndromes (PAD). Generally, patients with these types of immune conditions suffer acute and recurrent infections and are at an increased danger of malignancy and autoimmunity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibody Deficiency Syndromes and COVID-19
CVID is not a particular disease. Instead, it is a collection of hypogammaglobulinemia syndromes that occur due to multiple genetic defects. Researchers reported that CVID is found in 1 in every 25,000 individuals. It is considered to be the most common immunodeficiency in patients. Typically, individuals with PAD are subjected to intravenous or subcutaneous immunoglobulin replacement therapy that lowers the possibility of infection.
In response to the COVID-19 pandemic, scientists have designed effective vaccines against the spike protein of SARS-CoV-2.
As of today, two vaccines have received full approval from the US Food and Drug Administration (FDA), the mRNA-1273 (Moderna) and the BNT162b2 (Pfizer-BioNTech). The Ad26.COV2.S (Johnson & Johnson/Janssen) has received emergency use authorization (EUA).
Scientists have stated that not much evidence is available regarding the efficacy of COVID-19 mRNA or adenoviral vector vaccines against SARS-CoV-2 infection in CVID or PAD patients. A prior study indicated variable seroconversion rates, with the presence of anti-spike, S1, or RBD antibodies in 90% of PAD patients who were provided with BNT162b2, mRNA, or ChAdOx1 (Oxford-AstraZeneca) vaccine. This study further reported that better immune responses were found in patients with a prior history of SARS-CoV-2 infection. However, no evidence was found regarding the longevity of the immune protection of booster vaccination in PAD patients. Also, research is scarce in the context of the ability of PAD patient’s serum in neutralizing SARS-CoV-2 variants, particularly, the currently circulating SARS-CoV-2 Omicron variant.
A New Study
A new study posted to the medRxiv* preprint server has evaluated the effect of mRNA-based COVID-19 vaccination and boosting strategy on serum antibody responses in PAD patients against the original SARS-CoV-2 strain and circulating variants. The study revealed that although this group of patients has impaired humoral responses, vaccination played an essential role in protecting them from COVID-19 infection.
Researchers revealed that two doses of mRNA vaccines generated enough antibodies in most PAD patients to seemingly protect individuals against the original SARS-CoV-2 strain as well as the B.1.617.2 variant. Interestingly, researchers found that PAD patients with no history of COVID-19 infections exhibited little to no serum neutralizing ability against the Omicron variant following two vaccination doses. However, when this group was treated with an mRNA vaccine booster, an improved level of anti-Omicron response was observed in most individuals. In addition, researchers found that the levels of neutralizing antibodies declined over time.
The study's findings are in line with a recent report of the Center for Disease Control and Prevention (CDC) that suggests a three-dose primary mRNA vaccine for moderately or severely immune-suppressed individuals. Scientists found that the level of immune response among PAD patients with two doses of mRNA vaccines, without a history of COVID-19 infection, was lower in magnitude and less durable compared to those who recovered from SARS-CoV-2 infection and were vaccinated. They further observed an increase in serum neutralizing titers after booster vaccination than the increase in anti-spike and anti-RBD titers. This finding highlights the importance of conducting antibody neutralization assays in addition to spike or RBD binding assays while determining the quality of humoral immune responses.
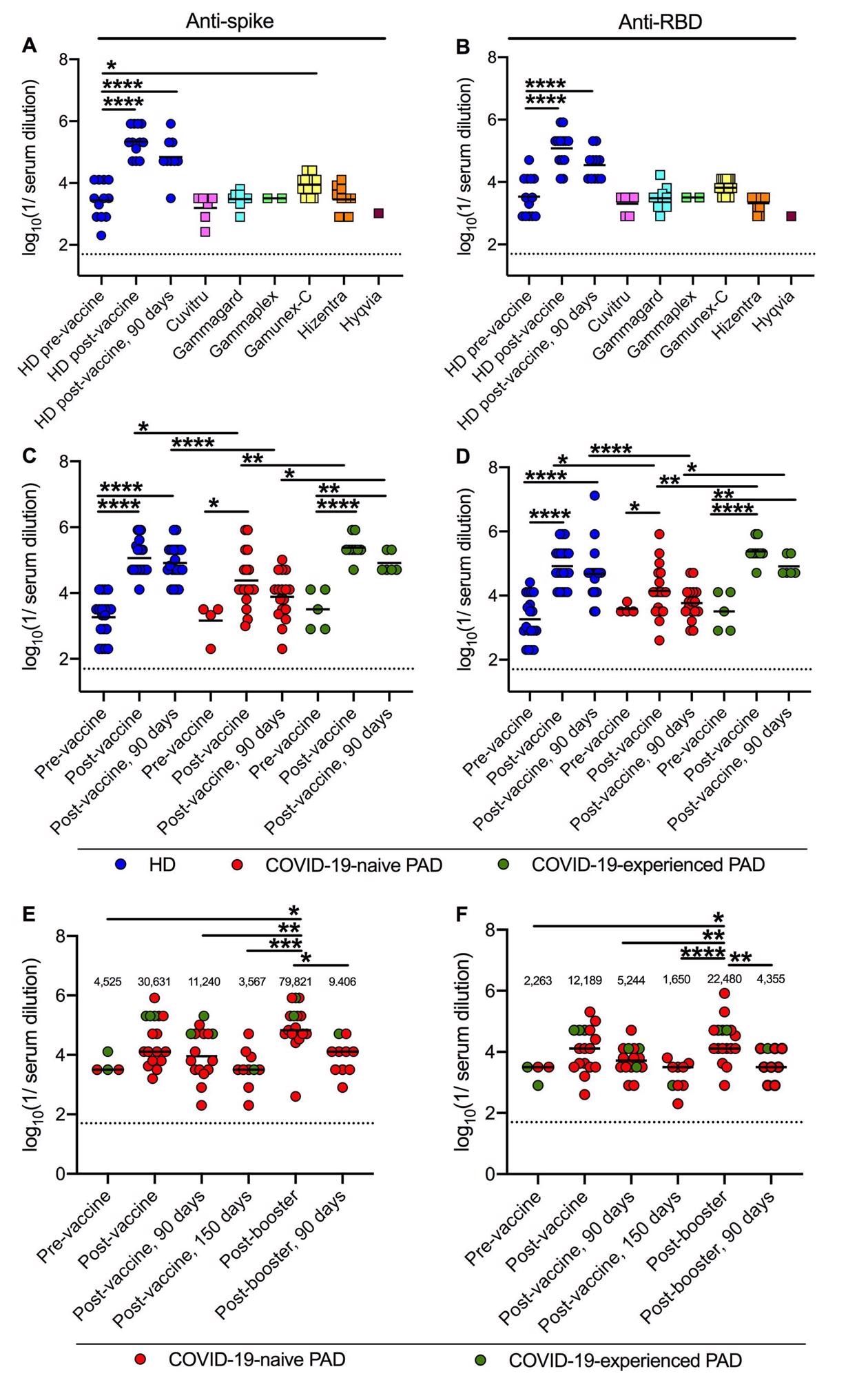
Anti-spike and anti-RBD titers following primary vaccination and boosting in PAD patients. Anti-Wuhan-1 Spike (A) and RBD (B) endpoint titers in 48 lots of 6 different immunoglobulin replacement products (squares) compared to 12 HD (blue circles) before, 14 days and 90 days post-completion of BNT162b2 vaccine series. Anti-Wuhan-1 spike (C) and RBD (D) endpoint titers in HD (n = 20; blue circles), COVID-19-naive (n = 18; red circles) and COVID-19-experienced (n = 9; green circles) PAD patients before, or 14 and 90 days post-completion of mRNA (BNT162b2, n = 19 or mRNA-1273, n = 8) vaccination series. Anti-Wuhan-1 Spike (E) and RBD (F) endpoint titers in COVID-19-naive (n = 14; red circles) and COVID-19-experienced (n = 3; green circles) PAD patients before (n = 4), 14 or 28 (n = 17), 90 (n = 16) and 150 (n = 10) days post-completion of primary mRNA (BNT162b2 n = 13, mRNA-1273 n = 2) or Ad26.COV2.S (n = 2) vaccine series, and 14 (n = 17) days and 90 (n = 10) days post-booster with mRNA vaccine (BNT162b2 n = 15; or mRNA-1273 n = 2). Dotted black line represents the limit of detection. Numbers above graphed data (E-F) represent the geometric mean titer (GMT) for each time point. One-way ANOVA with Dunnett’s post-test; Bars indicate mean values; Only significant differences are shown: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
In the future, further research is required to assess B cells in blood from PAD patients after they undergo antibody maturation after infection, vaccination, or boosting. The authors highlighted that one of the limitations of this study was the heterogeneity in the PAD patient cohort. The study cohort included individuals with CVID, hypogammaglobulinemia, or specific antibody deficiency. However, scientists did not observe any considerable difference in antibody responses to mRNA vaccines between patients. Interestingly, many patients who previously responded poorly to bacterial or other protein antigens (e.g., tetanus toxoid) reacted to mRNA vaccines. Although the precise reason for this difference in immune reaction is not clear, it could be due to the unique adjuvant properties of the lipid nanoparticle.
Researchers reported that no antibody response was detected after 90 days of immunization among COVID-naive PAD patients with the Ad26.COV2.S adenoviral-vectored vaccine. This study indicates that an mRNA-based vaccine may be more effective for PAD patients.
Conclusion
Scientists revealed that the neutralizing titers were below the recognized protective cut-off in the COVID-19-naive PAD patients. However, vaccinating PAD patients with mRNA vaccines along with boosters is an effective strategy to protect this group from SARS-CoV-2 and its variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zimmerman, O. et al. (2022) mRNA vaccine boosting enhances antibody responses against SARS-CoV-2 Omicron variant in patients with antibody deficiency syndromes, medRxiv, 2022.01.26.22269848; doi: https://doi.org/10.1101/2022.01.26.22269848, https://www.medrxiv.org/content/10.1101/2022.01.26.22269848v1
- Peer reviewed and published scientific report.
Zimmerman, Ofer, Alexa Michelle Altman Doss, Paulina Kaplonek, Chieh-Yu Liang, Laura A. VanBlargan, Rita E. Chen, Jennifer Marie Monroy, et al. 2022. “MRNA Vaccine Boosting Enhances Antibody Responses against SARS-CoV-2 Omicron Variant in Individuals with Antibody Deficiency Syndromes.” Cell Reports Medicine 3 (6). https://doi.org/10.1016/j.xcrm.2022.100653. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(22)00185-9.