The coronavirus disease 2019 (COVID-19) pandemic, which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has claimed more than 5.8 million lives worldwide. Several COVID-19 vaccines have received emergency use authorization (EUA) from regulatory agencies around the world, which has allowed almost 62% of the global population to be vaccinated against SARS-CoV-2.
Previous studies have revealed that vaccination has significantly reduced mortality, severe infection, and even transmission of SARS-CoV-2. Some of the COVID-19 vaccines that received EUA are Comirnaty® (BioNTech/Pfizer), Vaxzevria® (AstraZeneca), and Spikevax® (Moderna) vaccines.
Study: Allergological Study in Patients Vaccinated Against COVID-19 with Suspected Allergic Reactions. Image Credit: Microgen / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The pace of COVID-19 vaccination has suffered as a result of vaccine hesitancy and vaccine supply shortages. Vaccine hesitancy is driven by various reasons, such as fear of possible allergic reactions and negationism.
Some of the adverse effects following COVID-19 vaccination have occurred due to the activation of protective immune responses; however, these are not allergic reactions. Immediate hypersensitivity reactions have been attributed to the excipients polyethylene glycol (PEG) and/or polysorbate 80 (PS80), both of which are used in most vaccines.
It must be noted that the reactions to these compounds are very rare. Diagnoses of hypersensitivity to these polymers remains difficult, as skin tests are not yet standardized and their efficacy is not well established.
Previous studies have shown that cases of immediate or delayed hypersensitivity to SARS-CoV-2 vaccines are very rare. Furthermore, the etiopathogenic mechanism of allergic reactions to these vaccines remains unclear. Research has shown that anaphylaxis with COVID-19 vaccines is around 7.9 cases per million doses worldwide.
A new study that is currently posted to the Research Square* preprint server while under consideration for publication in BMC One Health Outlook, analyzes suspected reactions in patients vaccinated with the first and/or second dose of SARS-CoV-2 vaccines. Herein, the researchers also explored the cause of these reactions among their components and evaluated whether such reactions interfered with the vaccination protocol.
About the study
In the current study, researchers conducted a cross-sectional and descriptive study on patients who were suspected to demonstrate hypersensitivity to SARS-CoV-2 vaccines. The participants were subjected to a skin prick test (SPT) and/or intradermal test (IDT) with the vaccines and their excipients.
Histopathological and immunohistochemical studies were performed by skin biopsy in cases where patients were positive IDT with a particular vaccine. Finally, scientists also performed basophil activation tests (BAT) and lymphoblastic transformation tests (LTT).
Study findings
A total of sixteen patients were suspected to show hypersensitivity to the SARS-CoV-2 vaccine. Of these, twelve patients received Comirnaty®, three received Vaxzevria®, and one received Spikevax®.
Eight patients had immediate hypersensitivity reactions, while the remaining showed delayed reactions. The SPTs to excipients and vaccines were negative in all cases. Further, IDTs with all excipients were negative.
In two patients who were selected positive IDT with the vaccine, histological and immunohistochemical studies showed T-lymphocyte involvement. In both cases, BAT and LTT were negative.
In 44% of the patients studied, the vaccination protocol could be completed. The remaining 56% did not receive the second dose, either because they refused to be vaccinated or because they already had the complete regimen.
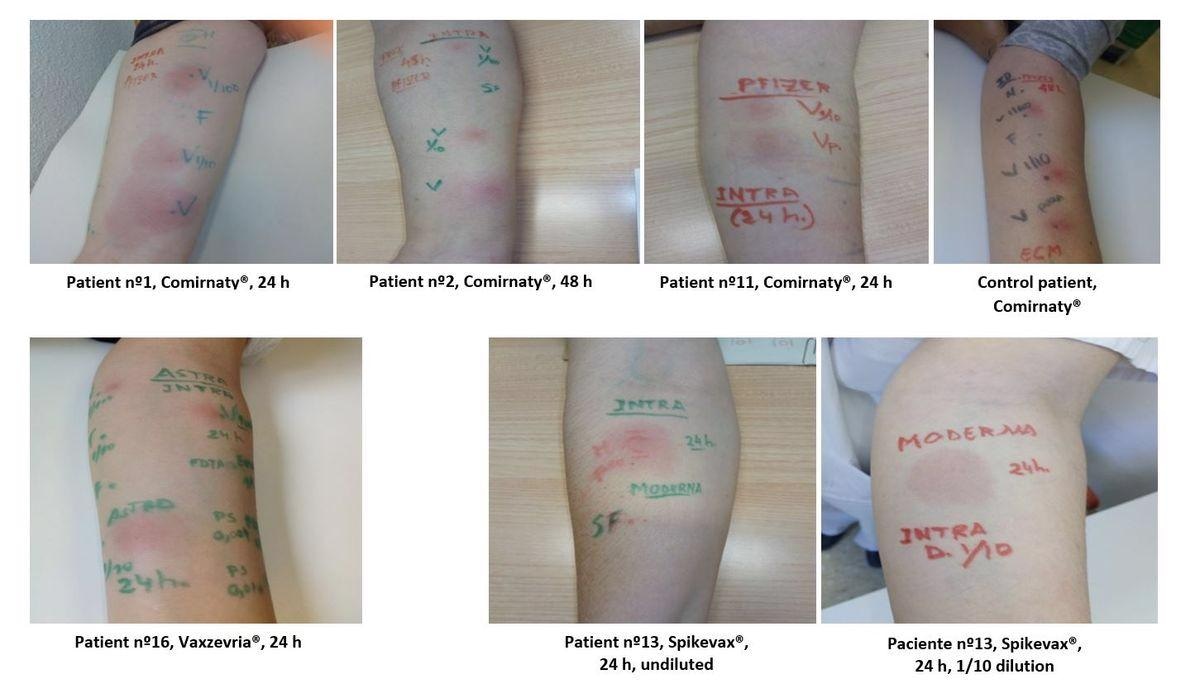
IDT with Comirnaty®, Vaxzevria® and Spikevax® F or SF: control with saline solution; Vp: undiluted vaccine; V1/100: 1/100 diluted vaccine; V1/10: 1/10 diluted vaccine
Strengths and limitations
The researchers stated that the important clinical idea is the main strength of this study. The objective of analyzing the impact of an interventional screening program in high-risk patients is extremely important to guide public health policies.
However, the results should be interpreted with caution because of the small sample size and the fact that all participants came from a very specific region. To gain a better understanding of the issue, studies with larger sample sizes and greater representation of all possible regions are needed.
Conclusions
The vaccination protocol could be successfully completed in approximately half of the patients who demonstrated possible hypersensitivity reactions to SARS-CoV-2 vaccines. This is largely because of this allergological and immunohistochemical study and is, therefore, a significant achievement.
In the future, IDTs with vaccines could be a potentially valuable method for assessing the immunogenicity of vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Cerdá, J. V., Pacheco, R. R., Witek, J. D., et al. (2022) Allergological Study in Patients Vaccinated Against COVID-19 with Suspected Allergic Reactions. Research Square. doi:10.21203/rs.3.rs-1301307/v1.
- Peer reviewed and published scientific report.
Jover Cerdá, Vicente, Ramón Rodríguez Pacheco, Joan Doménech Witek, Sonia Alonso Hernández, Rafael Durán García, Marina Real Panisello, and Francisco Manuel Marco de la Calle. 2022. “Allergological Study in Patients Vaccinated against COVID-19 with Suspected Allergic Reactions.” Allergy, Asthma & Clinical Immunology 18 (1). https://doi.org/10.1186/s13223-022-00685-z. https://aacijournal.biomedcentral.com/articles/10.1186/s13223-022-00685-z.