In order to infect human cells, SARS-CoV-2 uses the receptor-binding domain (RBD) to engage with the angiotensin-converting enzyme 2 (ACE2). Consequently, antibodies generated towards RBD after infection or vaccination can block this interaction with the ACE2, subsequently offsetting SARS-CoV-2 and halting the development of coronavirus disease 2019 (COVID-19).
This is why neutralizing antibodies are viewed as a strong correlate of protection for most viral vaccines, including SARS-CoV-2 vaccines, with IgM and IgG being widely implicated in the effective neutralization of the pandemic virus. But what about other antibodies in the human immune repertoire?
Throughout the COVID-19 pandemic, the study of IgA responses against SARS-CoV-2 has been relatively neglected. We know that IgA in the plasma peaks during acute infection and is somewhat dominant in that phase but relatively transient in nature.
This paper, first-authored by Dr. Samantha K Davis from the University of Melbourne in Victoria (Australia), aimed to examine purified fractions of plasma and purified IgA and IgG in order to appraise the contribution of these isotypes to the polyclonal convalescent antibody response to RBD and its neutralization capacity.
Determining the contribution of IgA to neutralization
A total of 41 SARS-CoV-2 convalescent subjects and 26 uninfected subjects donated their plasma samples for research purposes. A multiplex binding inhibition assay, highly comparable to the gold standard, was used to examine the neutralization properties of obtained plasma samples.
Furthermore, to determine the contribution of IgG and IgA to the neutralizing capacity of convalescent plasma, the researchers have depleted IgA from plasma and also depleted plasma of both IgA and IgG. This way, they assessed the propensity of antibody depleted plasma fractions to inhibit RBD binding to ACE2.
This research group has compared the ACE2 inhibitory capacity of matched purified IgG and IgA from the same individuals at equivalent total purified antibody concentrations. In addition, many other steps have been pursued to ascertain the importance of antibody titer for an individual's ability to facilitate IgA mediated ACE2 binding inhibition.
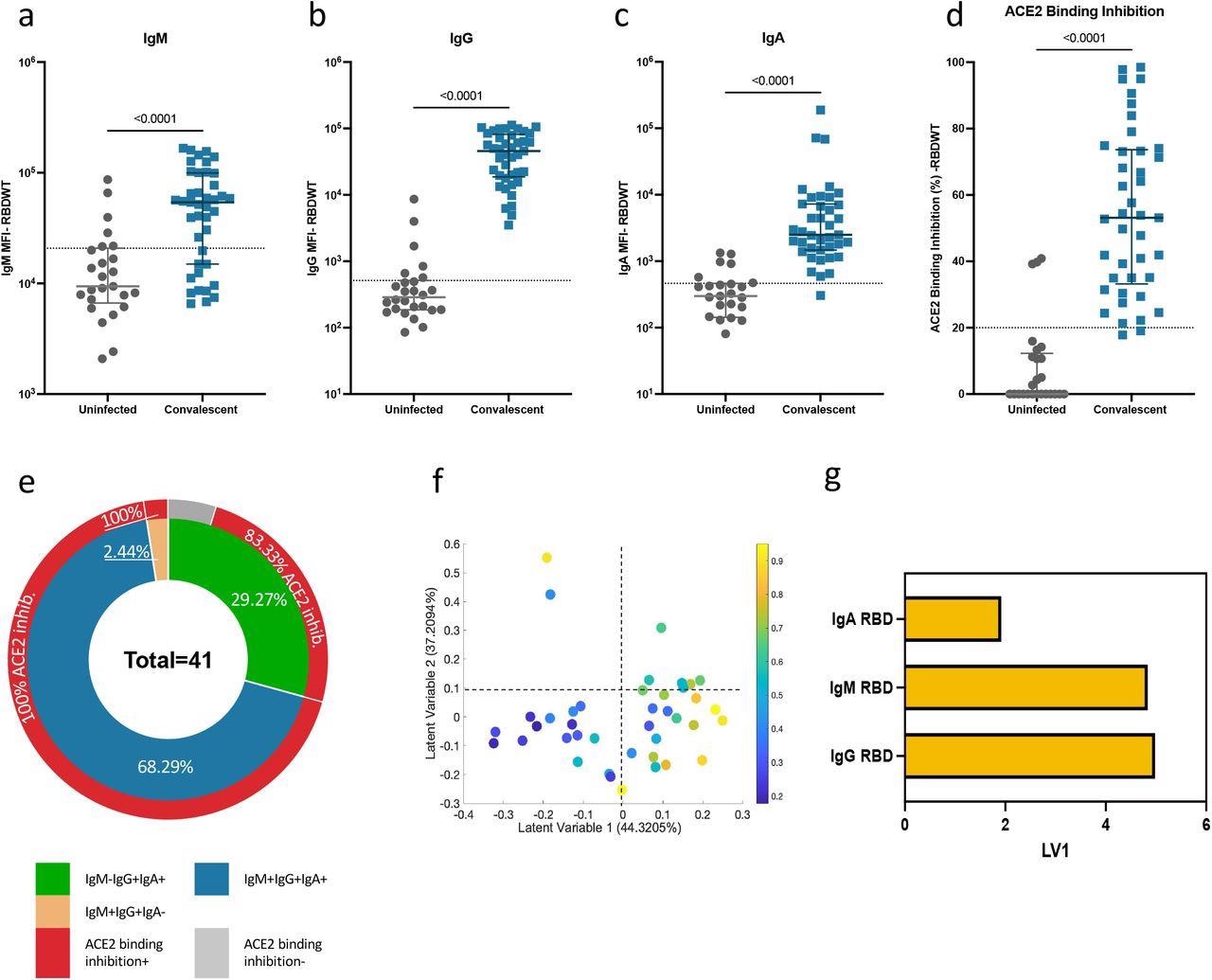
Convalescent plasma-induced robust anti-SARS-CoV-2 RBDWT antibody isotypes levels that inhibit ACE2 from binding RBDWT Convalescent (n-41) (blue) and uninfected control (n=26) (grey) plasma (final dilution 1:100) was assessed for IgM (a), IgG (b) and IgA (c) antibody binding to SARS-CoV-2 RBDWT via multiplex. A positive threshold (grey dotted line) was defined as the 75th percentile of antibody binding (MFI) for uninfected control plasma to respective antigens. (d) RBDWT ACE2 binding inhibition (%) of convalescent (blue) and uninfected control (grey) plasma (diluted 1:100). A positive threshold (grey dotted line) was defined as >20% ACE2 binding inhibition. (e) Pie chart outlining the percentage of subjects seropositive for anti-RBDWT antibody isotypes (IgM-IgG+IgA+ (green), IgM+IgG+IgA+ (blue), IgM+IgG+IgA-(yellow)) in the inner ring and the percentage of each seropositive subset with ACE2 binding inhibition in the outer red ring. Partial Least Squares Regression (PLSR) were conducted to determine the multivariate relationship between anti-RBD-specific isotype antibodies (IgG, IgA, and IgM) and % RBDWT-ACE2 binding inhibition (% ACE2 binding inhibition illustrated as a color gradient legend on the right-yellow-strongest to dark blue-weakest). PLSR Scores (f) and loadings plot (g). Percent variance for each latent variable (LV) in parentheses.
IgA can inhibit SARS-CoV-2 receptor binding
The results have shown that, during the early convalescence period, anti-RBD plasma IgA antibodies can contribute to the neutralizing capacity for many individuals, alongside IgG and IgM isotypes. More specifically, plasma IgA from more than 60% of the individual samples that have been included in the study shows the capacity to inhibit the interaction between RBD and ACE2.
In addition, a decreased neutralization effect that was observed when IgA was depleted from convalescent plasma supports this theory. For the first time, it was also shown that IgA depletion and IgG depletion also reduces neutralization for SARS-CoV-2 strains that harbor RBD mutations.
More specifically, plasma IgA and IgG antibodies from the test subjects broadly recognized kindred RBD epitopes and showed similar ability to inhibit ACE2 from binding 22 out of 23 different prevalent RBD proteins with a single amino acid mutation.
Implications for further analysis of vaccine response
Overall, this research group has demonstrated that convalescent plasma IgA recognizes not only RBD of the original SARS-CoV-2 strain but also a myriad of RBD mutants, with the ability to halt ACE2 engagement with RBD akin to IgG when sufficient IgA titers are induced.
"Furthermore, the convalescent IgA neutralizing response is highly heterogeneous between individuals, with a third of the cohort inducing stronger IgA-mediated inhibition of RBD engagement with ACE2 than IgG, when tested at equivalent concentrations", explain study authors in this medRxiv paper which is currently under peer review.
In any case, dissecting the IgA response in the context of vaccination against COVID-19, as well as how it responds to different variants of concern, will be pivotal in further addressing the importance of IgA in a protective polyclonal antibody response.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Davis, S.K. et al. (2022). Heterologous SARS-CoV-2 IgA neutralising antibody responses in convalescent plasma. medRxiv. https://doi.org/10.1101/2022.02.06.22270359, https://www.medrxiv.org/content/10.1101/2022.02.06.22270359v1
- Peer reviewed and published scientific report.
Davis, Samantha K, Kevin John Selva, Ester Lopez, Ebene R Haycroft, Wen Shi Lee, Adam K Wheatley, Jennifer A Juno, et al. 2022. “HeterologousSARS‐CoV‐2IgANeutralising Antibody Responses in Convalescent Plasma.” Clinical & Translational Immunology 11 (10). https://doi.org/10.1002/cti2.1424. https://onlinelibrary.wiley.com/doi/10.1002/cti2.1424.