
 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
While the population-based studies and clinical trials on COVID-19 vaccines have demonstrated a brilliant transitory safety and efficacy profile of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger ribonucleic acid (mRNA) vaccines, only a few participants aged 70 years or older were included in these trials. Yet, COVID-19-related mortality was higher in older subjects, particularly those with comorbidities.
The Canadian Advisory Committee on Immunization recommended increasing vaccine dosing intervals up to four months and mixing of brands to partly vaccinate more individuals against SARS-CoV-2 during a period of limited vaccine availability. These public health policies raised concerns regarding vaccine efficacy.
Although few investigations suggested the positive impact of extending dosing intervals on immune responses, the effects of the vaccine-elicited antibody responses within the elderly mobile population were undefined. Further, apart from clinical trials, proof regarding the efficacy of vaccination from real-world data and during breakthroughs is necessary to devise booster vaccination strategies.
About the study
In the present decentralized prospective cohort study, called the stopCoV study, the researchers compared the responses of the COVID-19 vaccine-induced antibodies among young and elderly participants. In addition, the team also compared the safety of the SARS-CoV-2 vaccines between these cohorts.
The study presents data regarding the self-reports of adverse events and self-collected dried blood spots (DBS) from 911 participants aged 70 years or older and 375 participants aged between 30 and 50 years up to 12 weeks of receiving the second vaccine dose.
Individuals who consented to contact for research received study information emails following SARS-CoV-2 vaccination at Ontario distribution centers. Similarly, the Ontario Canadian Association of Retired Persons members also received these emails. Further, subjects can also enroll through the study website, www.stopcov.ca., before the first or second dose vaccination. Participants were enrolled in the study following the submission of an electronic consent form.
The self-administered electronic survey forms collected data regarding baseline demographics and health. Electronic diaries obtained information on vaccine brand, vaccination dates, systemic and local adverse events up to seven days of each vaccine dose. The check-in questionnaires sent monthly procured information on adverse events lasting long term, booster doses, and the latest SARS-CoV-2 diagnoses.
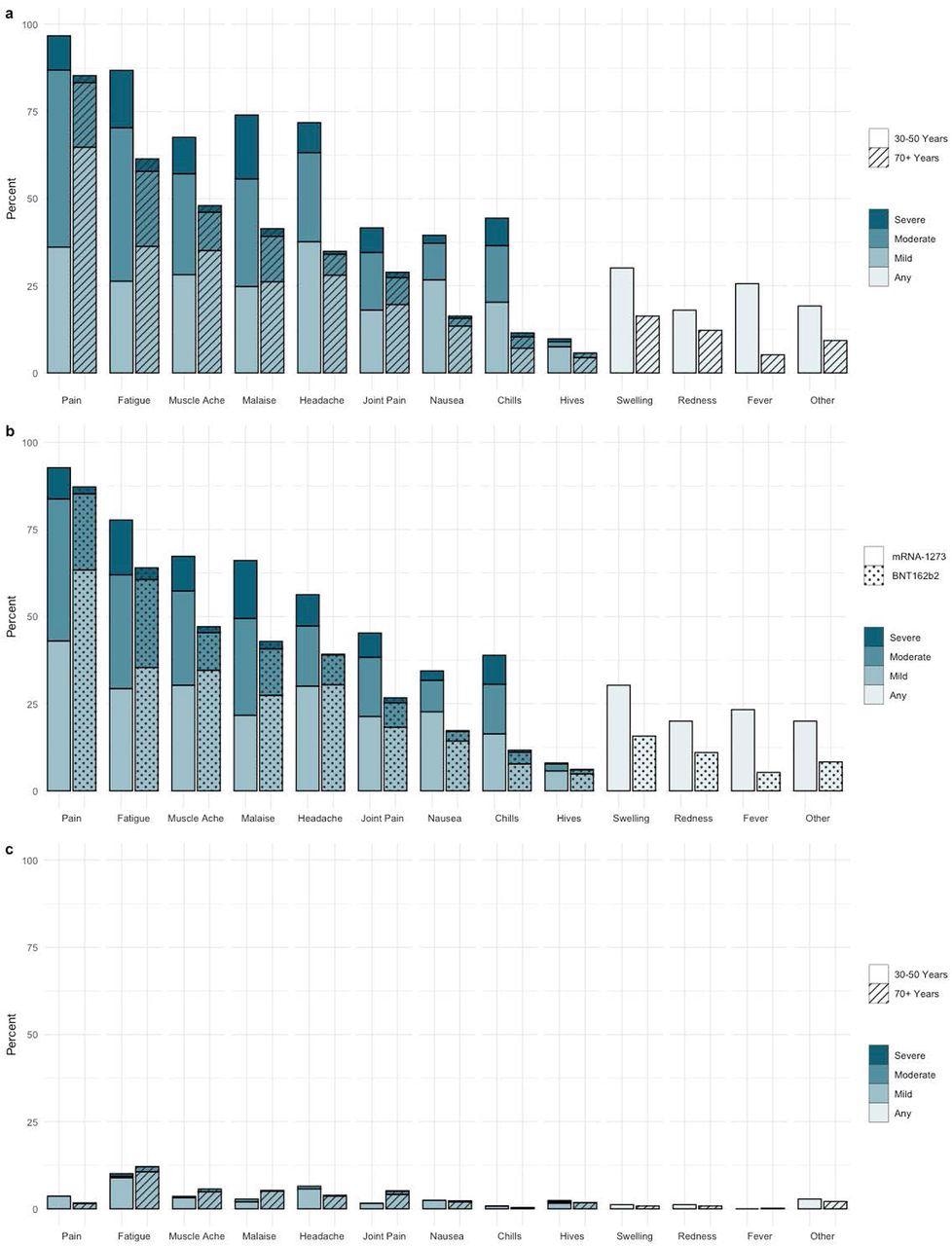
Self Reported Adverse events to Second vaccine dose a) Adverse events and reported severity during first 7 days post-dose 2 by age cohort (n=955) b) Adverse Events and reported severity during first 7 days post-dose 2 by vaccine type (n=938) c) Adverse Events and severity reported on day 7 post-dose 2 by age cohort (n=905)
Findings
The results indicate that nearly 95% of the study participants reported at least one adverse event following the SARS-CoV-2 vaccination, but they were mild and short-lived. These adverse events were more frequent in the younger subjects and the recipients of mRNA-1273 vaccines. However, few patients had residual symptoms after seven days of vaccination.
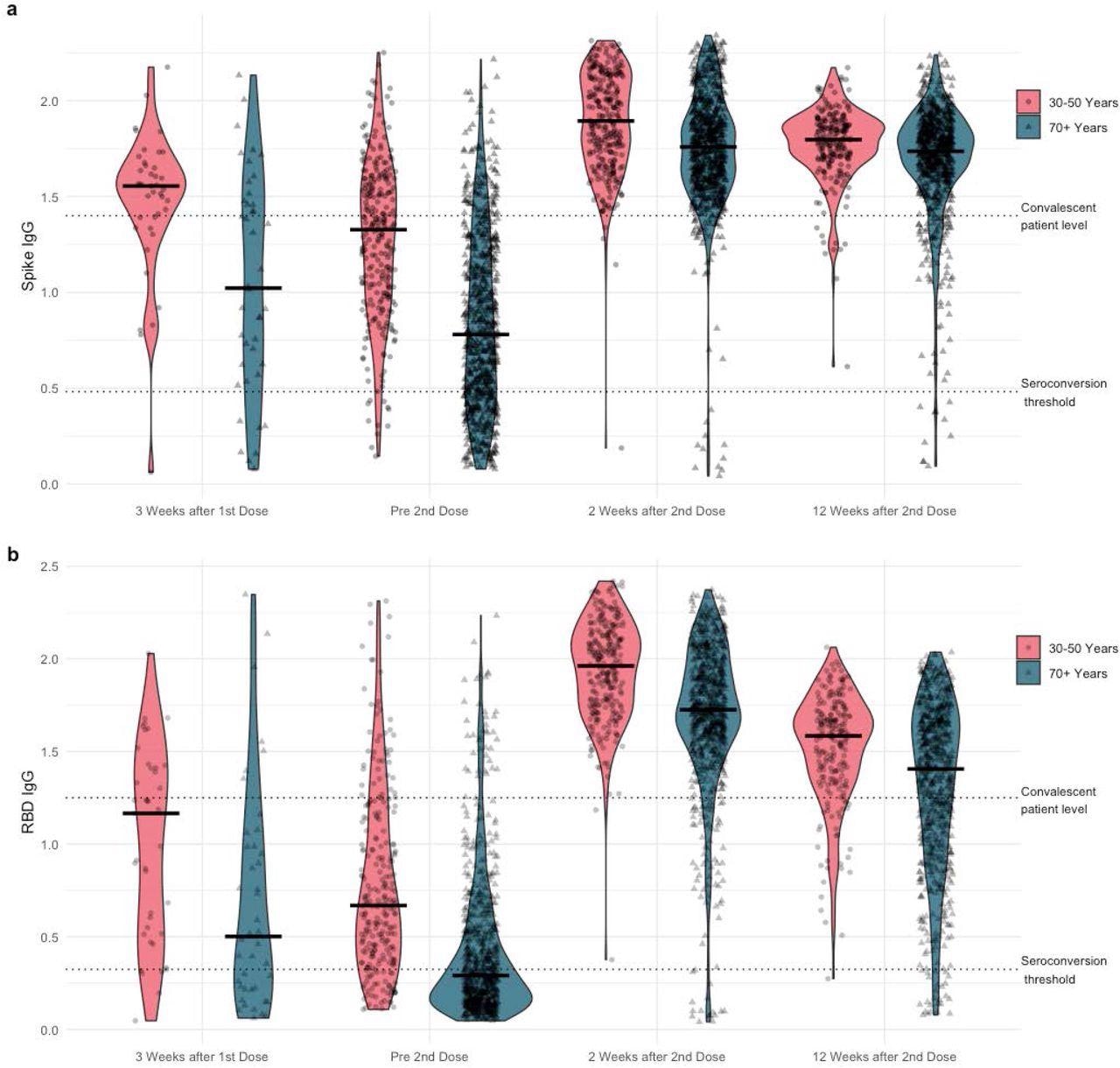
Violin plots of a) anti-Spike and b) anti-RBD (receptor binding domain) IgG normalized ratios by Time and by Age Cohort
The systemic and local SARS-CoV-2 reactivity rates, particularly in young participants, were high but transitory following the mRNA-1273 vaccination. After one dose of the mRNA-1273 vaccine, 84% of the young cohorts had positive IgG antibodies against SARS-CoV-2 spike (S) and receptor-binding domain (RBD) proteins, which increased to 100% following the second mRNA-1273 vaccination. However, only 46% of the older subjects developed positive immunoglobulin G (IgG) antibodies to SARS-CoV-2 S and RBD antigens which increased to 98% by the second dose of mRNA-1273 vaccine.
Low normalized IgG RBD antibody ratios after two weeks of the second dose of SARS-CoV-2 vaccination statistically correlated with male gender, old age, history of cancer, low body weight, cardiovascular disease, the BNT162b2 vaccine, immunosuppression, and long intervals between doses in the multivariable linear regression analysis.
Although lower antibody responses were observed each week before and after the second dose of SARS-CoV-2 vaccination, these effects were insignificant following 12 weeks of vaccination, after adjusting other covariates such as age and comorbidities.
The SARS-CoV-2 RBD IgG antibody ratios declined in younger and older cohorts after 12 weeks of the second dose of SARS-CoV-2 vaccination; however, ratios were higher in younger participants than the elderly.
Conclusions
In the current study, the researchers report the real-time IgG antibody responses to SARS-CoV-2 vaccines in older and younger cohorts. The study findings indicated that the SARS-CoV-2 antibody responses were higher in young participants than the elderly and the recipients of at least one mRNA-1273 dose. Although the immunity threshold was unknown, correlations between neutralizing and binding antibodies were strongly positive.
Future extensive prospective studies will provide deep insight into vaccination policies of the elderly since more information regarding correlates of protective immunity is available now.
Overall, the study sheds light on the age-associated limitations of the first and second dose of SARS-CoV-2 vaccine-induced antibody responses irrespective of other demographic variables, the elderly being an underrepresented group in the clinical trials of the COVID-19 vaccines. Further, the findings demonstrate that the BNT162b2 vaccines generate less robust antibody responses than the mRNA-1273 vaccine, probably because of the large number of antigens present in the mRNA-1273 vaccine and the safety of increasing the dosing intervals in younger cohorts.
Although a single dose of COVID-19 vaccines generates significant antibody responses against SARS-CoV-2, the study reinforces the requirement of two doses, especially in the elderly, without any delay.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Safety and Efficacy of Preventative COVID Vaccines: The StopCoV Study, Sharon Walmsley, Leah Szadkowski, Bradly Wouters, Rosemarie Clarke, Karen Colwill, Paula Rochon, Michael Brudno, Rizani Ravindran, Janet Raboud, Allison McGeer, Amit Oza, Christopher Graham, Amanda Silva, Dorin Manase, Laura Parente, Jacqueline Simpson, Roya Monica Dayam, Adrian Pasculescu, Anne-Claude Gingras, medRxiv, 2022.02.09.22270734; doi: https://doi.org/10.1101/2022.02.09.22270734, https://www.medrxiv.org/content/10.1101/2022.02.09.22270734v1