The SARS-CoV-2 pandemic has caused over 411 million infections and 5.81 million deaths to date. A handful of highly effective vaccines have been developed and deployed to target the SARS-CoV-2 spike protein. The recently emerged variant of concern (VoC) Omicron has the most significant number of mutations in the spike protein among all SARS-CoV-2 variants. Evidence from various studies has suggested reduced serum neutralization and a large number of breakthrough infections by the B.1.1.529 variant in vaccinated individuals.
 Study: Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice. Image Credit: ktsdesign / Shutterstock
Study: Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice. Image Credit: ktsdesign / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study design
Cell lines such as African green monkey Vero-TMPRSS2 (transmembrane protease, serine 2) and Vero-hACE2 (angiotensin-converting enzyme 2)-TMPRRS2 were used in the present study. The researchers used WA1/2020 recombinant virus strain with D614G substitution and B.1.1.529 viral isolate. Heterozygous K18-hACE2 C57BL/6J, 129S2, and BALB/c mice were used.
mRNA encoding Wuhan-Hu-1 (mRNA-1273) and SARS-CoV-2 S-2P or B.1.1.529 (mRNA-1273.529) was synthesized in vitro through an optimized RNA-polymerase-mediated transcription and encapsulated in lipid nanoparticle.
Viral antigens like a spike (S) and receptor-binding domain (RBD) protein from SARS-CoV-2 Wuhan-1 and B.1.1.529 were expressed in Expi293F cells. An enzyme-linked immunosorbent assay (ELISA) was performed through 96 well microtiter plates. Focus reduction neutralization test and pseudovirus neutralization assays were performed, and cytokine and chemokine protein measurement was done followed by lung histology.
Findings
The researchers observed that after two immunizations with the mRNA-1273 vaccine of K18-hACE2 mice, there was a robust antibody response against Wuhan-1 and B.1.1.529 spike proteins. At the 5µg dose, mean serum endpoints titers against spike and RBD proteins of Wuhan-1 and B.1.1.529 ranged from ~4,000,000 to 800,000 and 1,000,000 to 40,000, respectively. At 0.1 µg dose, there was a 10-fold decrease in serum IgG response against the spike and RBD protein. Notably, after mRNA-1273 vaccination, there were reduced antibody titers against B.1.1.529 RBD as compared to the Wuhan-1 RBD.
The mRNA-1273 vaccine at 5 µg dose induced high serum neutralizing antibody responses against B.1.1.529 and WA1/2020 D614G. However, for B.1.1.529, there was a nearly eight-fold decrease in geometric mean titers (GMTs) of neutralization. The mRNA-1273 vaccine (0.1 µg dose), showed nearly eight-fold and greater than 20 fold reduction in neutralizing activity against WA1/2020 D614G and B.1.1.529, respectively.
The researchers observed that in K18-hACE2 mice compared to the control mRNA vaccine, the mRNA-1273 vaccine at 0.1 and 5 µg dose prevented loss of weight at 6 dpi after WA1/2020 D614G infection; however, in B.1.1.529-challenged mice, there was no effect on weight loss.
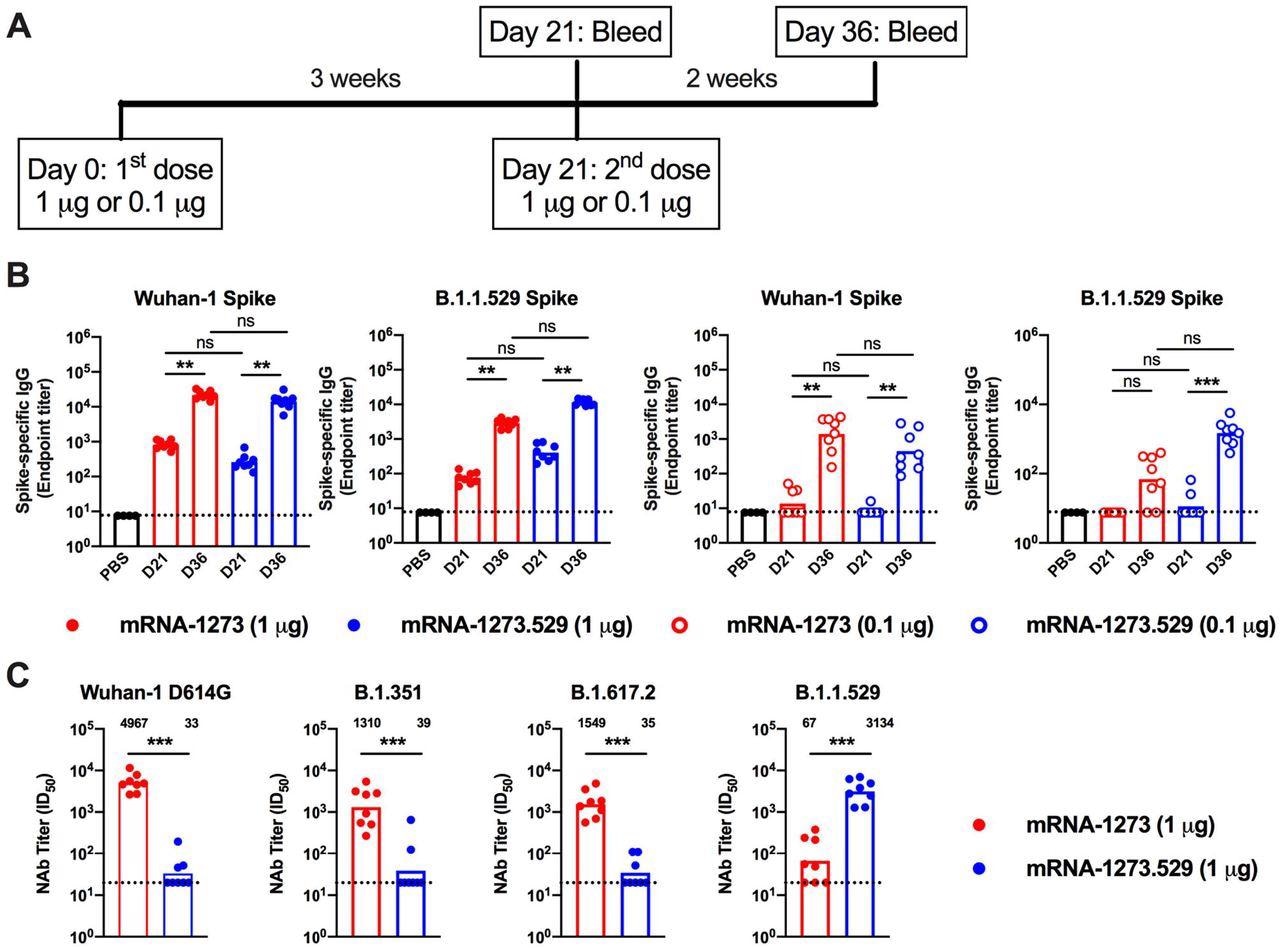
Antibody responses in BALB/c mice after immunization with mRNA-1273 and mRNA-1273.529 vaccines. Six-to-eight-week-old female BALB/c mice were immunized twice over a three-week interval with 1 μg of mRNA-1273 or mRNA-1273.529 vaccine or a PBS control (black circles). Immediately before (Day 21) or two weeks after (Day 36) the second vaccine dose, serum was collected. A. Scheme of immunization and blood draws. B. Serum antibody binding to Wuhan-1 or B.1.1.529 spike proteins by ELISA (n = 8, two experiments, boxes illustrate mean values, dotted lines show the LOD). C. Neutralizing activity of serum obtained two weeks after (Day 36) immunization with mRNA-1273 or mRNA-1273.529 vaccine against VSV pseudoviruses displaying the spike proteins of Wuhan-1 D614G, B.1.351 (Beta), B.1.617.2 (Delta), or B.1.1.529 (Omicron) (n = 8, two experiments, boxes illustrate geometric mean values, dotted lines show the LOD). GMT values are indicated above the columns.
In mRNA-vaccinated K18-hACE2 mice, levels of WA1/2020 D614G and B.1.1.529 infection was measured at 6 dpi. In the nasal washes of mice, there was a moderate amount of RNA for WA1/2020 D614G, and after the challenge with B.1.1.529, a 10-fold lower level was measured. In the nasal turbinates and lungs of control mRNA-vaccinated mice, there were approximately 100-fold and 10-fold lower levels of B.1.1.529 RNA compared to WA1/2020 D614G RNA.
In the respiratory tract samples, the mRNA-1273 vaccine at 5 µg dose protected against WA1/2020 D614G infection, while the presence of B.1.1.529 viral RNA in nasal washes or turbinates were not detected, indicating breakthrough infection in the lungs of the majority of animals.
For both viruses (WA1/2020 D614G and B.1.1.529), there was an inverse correlation with viral RNA amount in lungs, with higher infection observed in B.1.1.529-infected animals.
The researchers observed an increased expression of several pro-inflammatory cytokines and chemokines in mRNA-vaccinated K18-hACE2 mice infected with WA1/2020 D614G or B.1.1.529 in lung homogenates. At a 5-µg dose of the mRNA-1273 vaccine, levels of cytokines and chemokines were lower in the lungs of animals when challenged with WA1/2020 D614G or Omicron B.1.1.529. At a lower dose of 0.1 µg, there was a lower level of cytokines and chemokines in mice lungs with WA1/2020 D614G infection but not with the B.1.1.529 challenge.
Histological analysis of lung tissue of control mRNA-vaccinated mice challenged with WA1/2020 D614G showed pneumonia characterized by alveolar space consolidation, immune cell infiltration, interstitial edema, and vascular congestion; however, in B.1.1.529-challenged mice less lung pathology was observed, eliciting lower pathology of B.1.1.529 in rodents.
On the other hand, in mice immunized with high and low doses of mRNA-1273 and infected with WA1/2020 D614G, no lung pathology was observed. At a high dose of the mRNA-1273 vaccine, protection was elicited against B.1.1.529-infection-induced pathological changes and at a lower dose, similar lung pathology was observed as in control mRNA-vaccinated mice infected with B.1.1.529.
Mice boosted with mRNA-1273 had neutralizing titers against B.1.1.529 above the estimated threshold (titer of 50) for protection (GMT: 6,124, 5 μg; 1,161, 0.25 μg). High neutralization titers were observed against B.1.529 (GMT: 3, 314), lower levels of neutralization (85 to 100-fold less, P < 0.01) were detected against Wuhan-1 D614G (GMT: 33), B.1.351 (GMT: 39), and B.1.617.2 (GMT: 35), with most of the samples at the limit of detection of the neutralizing assay.
The administration of mRNA-1273 (5 or 0.25 μg dose) in mice showed increased levels (GMT: 29,161, 5 μg; 5,749, 0.25 μg) of pre-boost neutralizing antibodies against WA1/2020 N501Y/D614G. The researchers observed that both mRNA-1273 and mRNA-1273.529 boosters increased neutralizing activity of B.1.1.529; however, an Omicron-matched vaccine produced higher titers of neutralizing antibodies.
The researchers observed that in animals boosted with Omicron-matched mRNA-1273.529 vaccine, protection against B.1.1.529-induced inflammation of the lung was enhanced.
Conclusion
The study results showed that the administration of mRNA-1273 or Omicron-matched mRNA-1273.529 boosters elicited protection against Omicron infection in mice. Immunization with a low-dose series of the mRNA-1273 vaccine protected against WA1/2020 challenge, but there was a loss of neutralizing activity against B.1.1.529 due to breakthrough infection. The delivery of Omicron-matched and historical mRNA vaccines as boosters improved neutralization against B.1.1.529.
The findings suggested that boosting with historical vaccines and the Omicron variant-matched mRNA vaccine or heterologous platform targeting spike protein can minimize Omicron breakthrough infections by increasing neutralizing antibodies against SARS-CoV-2 or by enhancing antibody repertoire breadth to control variant strains.
The researchers warranted the need for further studies to evaluate the durability and magnitude of the boosted immune response, especially in vulnerable populations like the elderly, immunosuppressed, and immunocompromised.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Boosting with Omicron-matched or historical mRNA vaccines increases neutralizing antibody responses and protection against B.1.1.529 infection in mice. Baoling Ying, Suzanne M. Scheaffer, Bradley Whitener, Chieh-Yu Liang, Oleksandr Dmytrenko, Samantha Mackin, Kai Wu, Diana Lee, Laura E. Avena, Zhenlu Chong, James Brett Case, LingZhi Ma, Thu Kim, Caralyn Sein, Angela Woods, Andrea Carfi, Sayda M. Elbashir, Darin K Edwards, Larissa B. Thackray, Michael S. Diamond. bioRxiv 2022.02.07.479419, https://www.biorxiv.org/content/10.1101/2022.02.07.479419v1
- Peer reviewed and published scientific report.
Ying, Baoling, Suzanne M. Scheaffer, Bradley Whitener, Chieh-Yu Liang, Oleksandr Dmytrenko, Samantha Mackin, Kai Wu, et al. 2022. “Boosting with Variant-Matched or Historical MRNA Vaccines Protects against Omicron Infection in Mice.” Cell 185 (9): 1572-1587.e11. https://doi.org/10.1016/j.cell.2022.03.037. https://www.cell.com/cell/fulltext/S0092-8674(22)00387-7.