Oral antiviral medications, in addition to vaccines, are expected to play an important role in treating coronavirus disease 2019 (COVID-19), which is caused by infection with the severe acute respiratory disease coronavirus-2 (SARS-CoV-2).
These drugs must have significant antiviral activity, as well as target specificity, oral bioavailability, and metabolic stability. Although several antiviral compounds have been reported as possible SARS-CoV-2 inhibitors in vitro, only a few of these drugs have been shown to be effective in vivo.
Study: Oral administration of S-217622, a SARS-CoV-2 main protease inhibitor, decreases the viral load and accelerates recovery from clinical aspects of COVID-19. Image Credit: PHOTOCREO Michal Bednarek / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Ensitrelvir, a novel SARS-CoV-2 antiviral
Ensitrelvir (code name S-217622, brand name Xocova), is a new inhibitor of the SARS-CoV-2 major protease (Mpro), also known as 3C-like protease, has been shown to reduce the viral load and help alleviate the severity of SARS-CoV-2 in infected hamsters. In cells, low nanomolar to sub-micromolar doses of S-217622 suppress viral growth. In hamsters, oral treatment of S-217622 showed excellent pharmacokinetic qualities and hastened recovery from acute SARS-CoV-2 infection.
S-217622 also demonstrated antiviral effectiveness against SARS-CoV-2 variants of concern (VOCs), such as the highly pathogenic Delta variant and the newly discovered Omicron variant. Overall, these findings show that S-217622, which is an antiviral drug that is currently being tested in Phase II/III clinical trials, has impressive antiviral efficiency and effectiveness against SARS-CoV-2 and could be a viable oral treatment option for COVID-19.
Study findings
In a recent study published on the preprint server bioRxiv*, SARS-CoV-2 infected human bronchial epithelial cells (HBE) were treated with S-217622 in basal media. The progeny virus titers were reduced at small doses and fell below the plaque assay detection limit at 333 nM or more of S-217622. In the presence of S-217622, lactate dehydrogenase (LDH) production into the basal culture medium, which is a marker of virus cytotoxicity, was reduced.
S-217622 had antiviral activity comparable to or greater than antivirals that are currently approved for use in patients. Because nirmatrelvir and remdesivir are important substrates for the plasma membrane multidrug transporter P-glycoprotein (P-gp), they require a P-gp inhibitor like CP-100356 to prevent the antivirals from being effluxed from Vero cells, which have high levels of P-gp12. To this end, S-217622 suppresses SARS-CoV-2 infection in diverse cells at nanomolar to submicromolar doses.
Seven SARS-CoV-2 variants including the Alpha, Beta, Gamma, Delta, Omicron, Lambda, and Mu variants have been classified by the World Health Organization (WHO) as variants of concern (VOC) or variants of interest (VOI). These variants have amino acid alterations in the spike protein, which affect their infection rate, transmissibility, virulence, and susceptibility to neutralizing antibodies.
The nsp5 gene, which encodes Mpro, a target for S-217622, is largely conserved between each of these VOCs and VOIs, unlike the S gene, which encodes the viral spike protein. As a result of changes in the viral spike protein, S-217622 had antiviral effects against all VOCs, whereas neutralizing antibodies displayed varied reactivities to different VOCs. In Vero-TMPRSS2 cells infected with the SARS-CoV-2 Omicron variant, S-217622 retained exceptional antiviral effectiveness.
S-217622 exhibited antiviral activity in hamsters infected with various VOCs including the Alpha, Gamma, and Omicron variants. In hamsters, SARS-CoV-2 infection resulted in severe pneumonia and triggered various inflammatory responses; however, S-217622 treatment reduced inflammatory cytokine expression levels.
With SARS-CoV-2, hosts generate specific antibodies between 10 and 14 days after infection. In hamsters administered with S-217622, no seroconversion was observed.
These findings demonstrate that S-217622 has antiviral activity in vivo and that prophylactic treatment protects hamsters from infection with SARS-CoV-2. In the lungs of hamsters, prophylactic treatment of S-217622 led to a more than 105-fold reduction in viral ribonucleic acid (RNA) load and a virus titer below the detection limit at 4 days post-infection (dpi).
Furthermore, prophylactic treatment of S-217622 was found to prevent virus transmission among co-housed hamsters, thus demonstrating S-217622's strong in vivo antiviral activity.
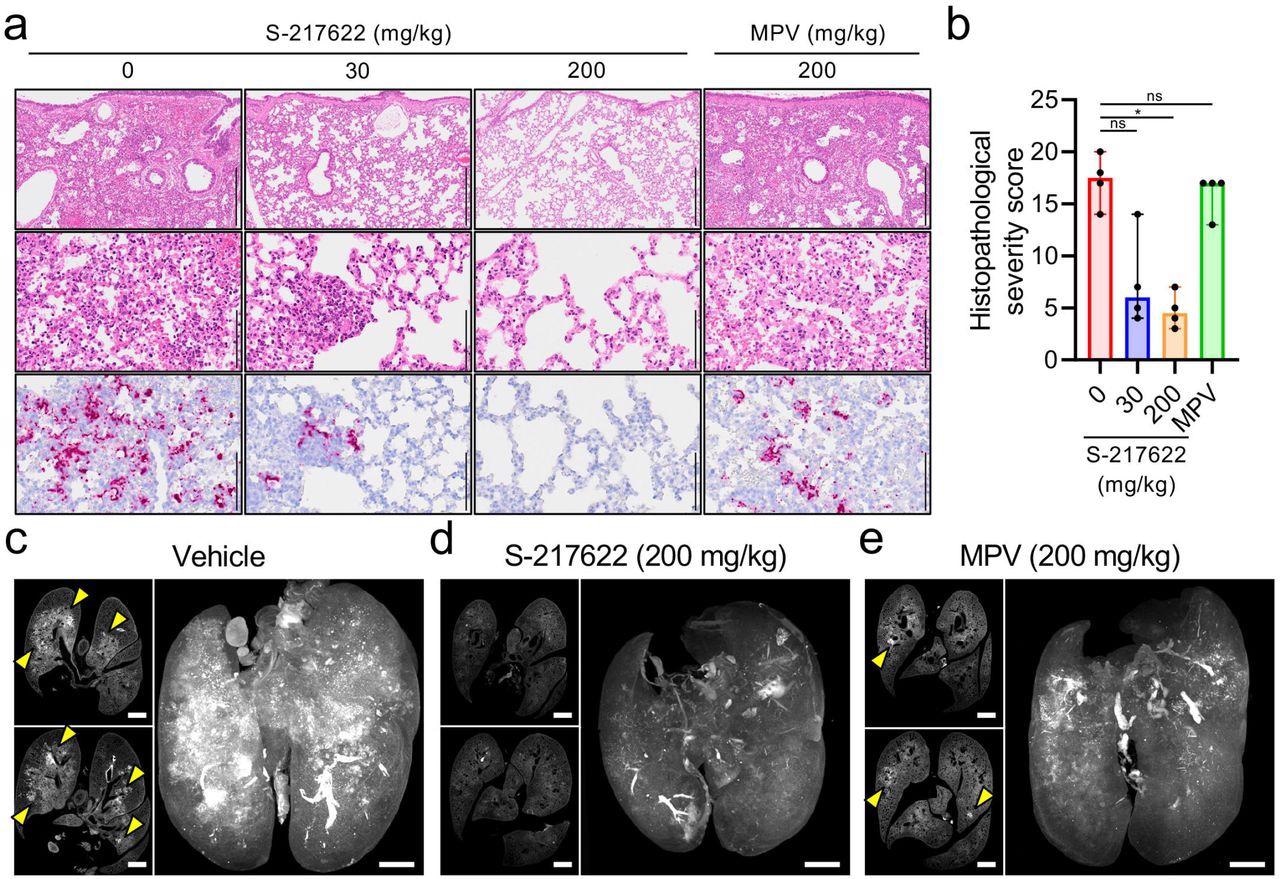
Histopathological findings in the lungs of SARS-CoV-2-infected hamsters that were administered antivirals The hamsters were infected with the SARS-CoV-2 Delta variant and sacrificed at 4 dpi for histopathological examinations. S-217622 (30 mg/kg or 200 mg/kg), molnupiravir (200 mg/kg), or vehicle control (0 mg/ml) was administered from 0 to 3 dpi (a, b) or 1 to 3 dpi (c-e) twice a day following the schedules shown in Fig. 2b and 3a, respectively. a, Representative histopathological images for the lung sections obtained from the animals given antivirals indicated above each panel (n = 4). Upper and middle panels, hematoxylin and eosin (H&E) staining. Lower panels, in situ hybridization (ISH) targeting the nucleocapsid gene of SARS-CoV-2. Scale bars in upper panels, 500 μm. Scale bars in middle and lower panels, 100 μm. b, Histopathological severity score of pneumonia based on the percentage of alveolitis area in a given section. Data are shown as the median score ± 95% confidential interval with each dot representing the score of each animal. (n = 4; ns = not significant, *p<0.05 by Kruskal-Wallis test with Dunn’s test.) c-e, Cross-sectional imaging of lungs and 3D image reconstruction of whole lungs from hamsters at 4 dpi. The infected hamsters were treated with vehicle (c), S-217622 (d), or MPV (e). The whole lung tissues were stained with anti-SARS-CoV-2 spike antibody and scanned by light sheet microscopy. Arrowheads indicate foci of SARS-CoV-2-positive alveoli. Scale bars, 2 mm.
Implications
The viral spike protein, which develops amino acid changes across SARS-CoV-2 variants, is the target of current vaccines and monoclonal antibody treatments. Comparatively, viral Mpro is less diverse, thereby demonstrating that the susceptibility of SARS-CoV-2 VOCs to S-217622 is likely comparable.
Taken together, the SARS-CoV-2 Mpro is a promising target for antiviral therapies. Furthermore, the findings from the current study demonstrate that S-217622 has a unique potential in treating COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sasaki, M., Tabata, K., Kishimoto, M., et al. (2022). Oral administration of S-217622, a SARS-CoV-2 main protease inhibitor, decreases the viral load and accelerates recovery from clinical aspects of COVID-19. bioRxiv. doi:10.1101/2022.02.14.480338. https://www.biorxiv.org/content/10.1101/2022.02.14.480338v1.full.
- Peer reviewed and published scientific report.
Sasaki, Michihito, Koshiro Tabata, Mai Kishimoto, Yukari Itakura, Hiroko Kobayashi, Takuma Ariizumi, Kentaro Uemura, et al. 2023. “S-217622, a SARS-CoV-2 Main Protease Inhibitor, Decreases Viral Load and Ameliorates COVID-19 Severity in Hamsters.” Science Translational Medicine 15 (679). https://doi.org/10.1126/scitranslmed.abq4064. https://www.science.org/doi/10.1126/scitranslmed.abq4064.